Kir7.1 is the physiological target for hormones and steroids that regulate uteroplacental function
- PMID: 40043131
- PMCID: PMC11881918
- DOI: 10.1126/sciadv.adr5086
Kir7.1 is the physiological target for hormones and steroids that regulate uteroplacental function
Abstract
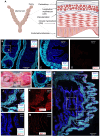
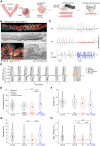
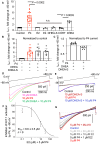
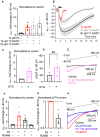
Preterm birth is detrimental to the well-being of both the mother and the newborn. During normal gestation, the myometrium is maintained in a quiescent state by progesterone. As a steroid hormone, progesterone is thought to modify uterine and placental morphology by altering gene expression, but another direct mode of action has long been suspected. Here, we reveal the nongenomic molecular mechanism of progesterone as the activation of human and murine inwardly rectifying potassium channel Kir7.1, which is expressed in myometrium and placental pericytes during late gestation. Kir7.1 is also activated by selective steroids, including those used to prevent premature labor, such as 17-α-hydroxyprogesterone caproate and dydrogesterone, revealing their unexpected mode of action. Our results reveal that Kir7.1 is the molecular target of both endogenous and synthetic steroids that control uterine excitability and placental function. Kir7.1, therefore, is a promising therapeutic target to support healthy pregnancy during mid and late gestation.
Figures

References
-
- What is the strongest muscle in the human body (2019/updated 2024); www.loc.gov/everyday-mysteries/biology-and-human-anatomy/item/what-is-th....
-
- Wray S., Arrowsmith S., Uterine excitability and ion channels and their changes with gestation and hormonal environment. Annu. Rev. Physiol. 83, 331–357 (2021). - PubMed
-
- Wray S., Noble K., Sex hormones and excitation-contraction coupling in the uterus: The effects of oestrous and hormones. J. Neuroendocrinol. 20, 451–461 (2008). - PubMed
-
- Khan-Dawood F. S., Dawood M. Y., Estrogen and progesterone receptor and hormone levels in human myometrium and placenta in term pregnancy. Am. J. Obstet. Gynecol. 150, 501–505 (1984). - PubMed
-
- Astle S., Slater D. M., Thornton S., The involvement of progesterone in the onset of human labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 108, 177–181 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

