Epstein-Barr virus infection promotes T cell dysregulation in a humanized mouse model of multiple sclerosis
- PMID: 40043135
- PMCID: PMC11881922
- DOI: 10.1126/sciadv.adu5110
Epstein-Barr virus infection promotes T cell dysregulation in a humanized mouse model of multiple sclerosis
Abstract
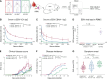
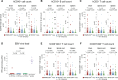
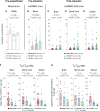
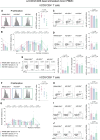
Latent infection with Epstein-Barr virus (EBV) is a strong risk factor for the development of multiple sclerosis (MS), although the underlying mechanisms remain unclear. To investigate this association, we induced experimental autoimmune encephalomyelitis (EAE) in immunodeficient mice reconstituted with peripheral blood mononuclear cells (PBMCs) from individuals with or without a history of EBV infection and/or relapsing MS (RRMS). HuPBMC EAE mice generated from EBV-seronegative healthy donors were less susceptible to developing severe neurological symptoms than healthy EBV-seropositive and RRMS donor groups. Donor EBV seropositivity and RRMS diagnosis were associated with a significant increase in the number of central nervous system (CNS) infiltrating effector T cells due to enhanced proliferation of proinflammatory T cells and limited expansion of regulatory T cells. The data indicate that a history of EBV infection, further compounded by a diagnosis of RRMS, promotes T cell-mediated xenogeneic CNS disease in a humanized mouse model of MS.
Figures



References
-
- Dendrou C. A., Fugger L., Friese M. A., Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015). - PubMed
-
- Compston A., Coles A., Multiple sclerosis. Lancet 372, 1502–1517 (2008). - PubMed
-
- Olsson T., Barcellos L. F., Alfredsson L., Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36 (2017). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

