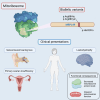
Bi-allelic variants in MRPL49 cause variable clinical presentations, including sensorineural hearing loss, leukodystrophy, and ovarian insufficiency
- PMID: 40043708
- PMCID: PMC12081275
- DOI: 10.1016/j.ajhg.2025.02.005
Bi-allelic variants in MRPL49 cause variable clinical presentations, including sensorineural hearing loss, leukodystrophy, and ovarian insufficiency
Abstract
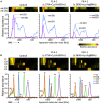
Combined oxidative phosphorylation deficiency (COXPD) is a rare multisystem disorder that is clinically and genetically heterogeneous. Genome sequencing identified bi-allelic MRPL49 variants in individuals from nine unrelated families with presentations ranging from Perrault syndrome (primary ovarian insufficiency and sensorineural hearing loss) to severe childhood onset of leukodystrophy, learning disability, microcephaly, and retinal dystrophy. Complexome profiling of fibroblasts from affected individuals revealed reduced levels of the small mitochondrial ribosomal subunits and a more pronounced reduction of the large mitochondrial ribosomal subunits. There was no evidence of altered mitoribosomal assembly. The reductions in levels of oxidative phosphorylation (OXPHOS) enzyme complexes I and IV are consistent with a form of COXPD associated with bi-allelic MRPL49 variants, expanding the understanding of how disruption of the mitochondrial ribosomal large subunit results in multisystem phenotypes.
Keywords: MRPL49; Perrault syndrome; combined oxidative phosphorylation deficiency; learning disability; leukodystrophy; mitochondria; mitoribosome; primary ovarian insufficiency; rare disease; sensorineural hearing loss.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Update of
-
Biallelic variants in MRPL49 cause variable clinical presentations, including sensorineural hearing loss, leukodystrophy, and ovarian insufficiency.medRxiv [Preprint]. 2024 Oct 11:2024.10.10.24315152. doi: 10.1101/2024.10.10.24315152. medRxiv. 2024. Update in: Am J Hum Genet. 2025 Apr 03;112(4):952-962. doi: 10.1016/j.ajhg.2025.02.005. PMID: 39417135 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

