Long-term safety and efficacy of anti-GM-CSF otilimab in patients with rheumatoid arthritis: long-term extension of three phase 3 randomised trials (contRAst X)
- PMID: 40044198
- PMCID: PMC11883618
- DOI: 10.1136/bmjopen-2024-088869
Long-term safety and efficacy of anti-GM-CSF otilimab in patients with rheumatoid arthritis: long-term extension of three phase 3 randomised trials (contRAst X)
Abstract
Objectives: To investigate the long-term safety and efficacy of otilimab, an antigranulocyte-macrophage colony-stimulating factor monoclonal antibody, for patients with rheumatoid arthritis (RA).
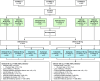
Methods: ContRAst X (NCT04333147) was a phase 3, multicentre, long-term extension trial. Patients with RA aged ≥18 years who completed a qualifying contRAst trial (contRAst 1-3) and who the investigator thought might benefit from long-term otilimab treatment were eligible to enter contRAst X. Patients who received otilimab (90 mg/150 mg) in their qualifying trial maintained the same dose; patients who received tofacitinib or sarilumab were rerandomised 1:1 to either otilimab dose. Patients could continue background conventional synthetic disease-modifying antirheumatic drugs. The primary objective was long-term safety (up to 4 years).
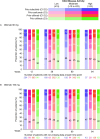
Results: Of the 2916 patients who entered contRAst X, 2915 received otilimab (exposure range: 7-896 days); the majority were withdrawn due to early trial termination. For otilimab 90 mg and 150 mg, the incidence of adverse events (AEs) was 62% (n=902/1456) and 64% (n=931/1459), the incidence of AEs of special interest was 8% (n=120/1456) and 7% (n=95/1459) and the incidence of serious AEs was 8% (n=123/1456) and 8% (n=114/1459), respectively. There were no instances of pulmonary alveolar proteinosis (PAP), active tuberculosis (TB), TB reactivation or serious hypersensitivity reactions. The proportions of clinical disease activity index low disease activity responders remained relatively stable throughout, with no apparent reduction following the switch from tofacitinib/sarilumab to otilimab.
Conclusion: No new safety signals or instances of PAP were associated with long-term (≤2.5 years) treatment with otilimab.
Trial registration number: ClinicalTrials.gov: NCT04333147.
Keywords: Clinical Trial; RHEUMATOLOGY; THERAPEUTICS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MEW receives research support from AbbVie, Aqtual, Bristol Myers Squibb and Lilly and consultation fees from AbbVie, Aclaris, Amgen, Bayer, Bristol Myers Squibb, CorEvitas, Genosco, Gilead Sciences, GSK, Horizon, Johnson & Johnson, Lilly, Novartis, Pfizer, Rami Therapeutics, R Pharma, Roche, Sanofi, Scipher, Sci Rhom, Set Point and Tremeau. He holds stock/stock options in CanFite, Inmedix and Scipher. PCT has received consulting fees from AbbVie, AnaptysBio, Acelyrin, Biogen, Immunovant, Fresenius, Galapagos, Gilead Sciences, GSK, Janssen, Lilly, Nordic Pharma, Pfizer, Roche, Sanofi and UCB and research support from Galapagos. IBMcI has received consultancy and research support from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Causeway, Compugen, Gilead Sciences, GSK, Lilly, Novartis, Pfizer and UCB and holds a leadership role in the University of Glasgow, Versus Arthritis and is an NHS GGC Board Member and an Annals of the Rheumatic Diseases Editorial Board Member. TA has accepted research grants and/or honoraria for meetings from AbbVie, Alexion, Astellas Pharma, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, GSK, Lilly Japan, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Takeda Pharmaceutical and UCB Japan. VS has received consulting fees from AbbVie, Alpine, Alumis, Amgen, Aria, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Ermium, Genentech/Roche, GSK, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Merck, MiMedx, Novartis, Omeros, Pfizer, R-Pharm, RAPT, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix, Tonix and Urica. TT received payment or honoraria from AbbVie, Asahi Kasei, Astellas, AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, Janssen, Lilly Japan, Mitsubishi-Tanabe and Pfizer Japan and is an Annals of the Rheumatic Diseases Editorial Board Member. MB, DB, PC, AG, RN, CO’S, DS, CS, MW and RW are employees of GSK and hold GSK stock/shares. KR is an employee of Probabilitas Consulting Limited and is contracted by GSK. RMF has received research support from AbbVie, Amgen, Arthrosi, AstraZeneca, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Galvani, Genentech/Roche, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung, Sanofi-Genzyme, Selecta and UCB; consulting fees from AbbVie, Amgen, Arthrosi, Bristol Myers Squibb, Cyxone, Dren Bio, Galapagos, Galvani, Gilead, GSK, Immunovant, ImmuneMed, InventisBio, Janssen, Kiniksa, Lilly, Monte Rosa, Novartis, Pfizer, Priovant, Recor, Samsung, Synact, UCB and VYNE and is an Annals of the Rheumatic Diseases Editorial Board Member.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
