Regio- and Enantioconvergent Hydroallylation of Acrylates Enabled by γ-Silyl-Substituted Allyl Acetates
- PMID: 40044597
- PMCID: PMC12051904
- DOI: 10.1002/anie.202425256
Regio- and Enantioconvergent Hydroallylation of Acrylates Enabled by γ-Silyl-Substituted Allyl Acetates
Abstract
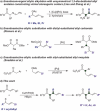
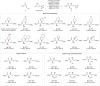
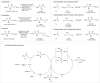
Transition-metal-catalyzed asymmetric allylic substitution provides an efficient route to chiral organic molecules featuring an allyl moiety, key intermediates in the synthesis of biologically active compounds. However, the use of unsymmetrical 1,3-disubstituted allyl electrophiles has been severely constrained by the challenges of achieving both regio- and stereoselectivity simultaneously. Herein, we present γ-silyl-substituted allyl acetates as highly effective electrophiles for a regio- and enantioconvergent hydroallylation, enabling the construction of vicinal stereogenic centers. This method delivers allylated products in 44%-93% yield with 79:21 to >95:5 dr and 88% to >99% ee. Additionally, the silyl group in the products can be readily converted into other functional groups, such as acyl and aryl groups, enhancing their synthetic utility.
Keywords: Copper; Enantioconvergent catalysis; Hydroallylation; Palladium; Vinylsilanes.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

