Virtual library docking for cannabinoid-1 receptor agonists with reduced side effects
- PMID: 40044644
- PMCID: PMC11882969
- DOI: 10.1038/s41467-025-57136-7
Virtual library docking for cannabinoid-1 receptor agonists with reduced side effects
Abstract
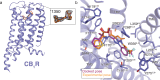
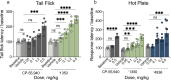
Virtual library docking can reveal unexpected chemotypes that complement the structures of biological targets. Seeking agonists for the cannabinoid-1 receptor (CB1R), we dock 74 million tangible molecules and prioritize 46 high ranking ones for de novo synthesis and testing. Nine are active by radioligand competition, a 20% hit-rate. Structure-based optimization of one of the most potent of these (Ki = 0.7 µM) leads to '1350, a 0.95 nM ligand and a full CB1R agonist of Gi/o signaling. A cryo-EM structure of '1350 in complex with CB1R-Gi1 confirms its predicted docked pose. The lead agonist is strongly analgesic in male mice, with a 2-20-fold therapeutic window over hypolocomotion, sedation, and catalepsy and no observable conditioned place preference. These findings suggest that unique cannabinoid chemotypes may disentangle characteristic cannabinoid side-effects from analgesia, supporting the further development of cannabinoids as pain therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.K.S. is co-founder of BlueDolphin, LLC, Epiodyne, and Deep Apple Therapeutics, Inc., and serves on the SRB of Genentech, the SABs of Schrodinger LLC and of Vilya Therapeutics. J.J.I. is a co-founder of BlueDolphin LLC and of Deep Apple Therapeutics. Y.S.M. is a VP of Sales and Marketing at Enamine Ltd. and a scientific advisor at Chemspace LLC. D.S.R. is an employee of Enamine, Ltd. H.K., C.N., M.S., and L.S. are employees of Domain Therapeutics North America Inc. The authors declare no other competing interests.
Figures





Update of
-
Large library docking for cannabinoid-1 receptor agonists with reduced side effects.bioRxiv [Preprint]. 2024 Feb 28:2023.02.27.530254. doi: 10.1101/2023.02.27.530254. bioRxiv. 2024. Update in: Nat Commun. 2025 Mar 06;16(1):2237. doi: 10.1038/s41467-025-57136-7. PMID: 38328157 Free PMC article. Updated. Preprint.
References
-
- Lipman, A. G. Medical cannabis for pain: anecdote or evidence. J. Pain. Palliat. Care Pharmacother.31, 96–97 (2017). - PubMed
-
- Iliopoulos-Tsoutsouvas, C., Georgiadis, M.-O., Ji, L., Nikas, S. P. & Makriyannis, A. Natural Compounds and Synthetic Drugs to Target Type-1 Cannabinoid (CB1) Receptor. in New Tools to Interrogate Endocannabinoid Signalling: From Natural Compounds to Synthetic Drugs (ed. Maccarrone, M.) 48–88 (The Royal Society of Chemistry). 10.1039/9781839160752-00048 (2020).
-
- Cohen, K., Weizman, A. & Weinstein, A. Positive and negative effects of cannabis and cannabinoids on health. Clin. Pharmacol. Ther.105, 1139–1147 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

