Analysis of human urinary extracellular vesicles reveals disordered renal metabolism in myotonic dystrophy type 1
- PMID: 40044661
- PMCID: PMC11882899
- DOI: 10.1038/s41467-025-56479-5
Analysis of human urinary extracellular vesicles reveals disordered renal metabolism in myotonic dystrophy type 1
Abstract
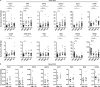
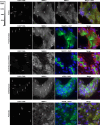
Chronic kidney disease (CKD) and the genetic disorder myotonic dystrophy type 1 (DM1) each are associated with progressive muscle wasting, whole-body insulin resistance, and impaired systemic metabolism. However, CKD is undocumented in DM1 and the molecular pathogenesis driving DM1 is unknown to involve the kidney. Here we use urinary extracellular vesicles (EVs), RNA sequencing, droplet digital PCR, and predictive modeling to identify downregulation of metabolism transcripts Phosphoenolpyruvate carboxykinase-1, 4-Hydroxyphenylpyruvate dioxygenase, Dihydropyrimidinase, Glutathione S-transferase alpha-1, Aminoacylase-1, and Electron transfer flavoprotein B in DM1. Expression of these genes localizes to the kidney, especially the proximal tubule, and correlates with muscle strength and function. In DM1 autopsy kidney tissue, characteristic ribonuclear inclusions are evident throughout the nephron. We show that urinary organic acids and acylglycines are elevated in DM1, and correspond to enzyme deficits of downregulated genes. Our study identifies a previously unrecognized site of DM1 molecular pathogenesis and highlights the potential of urinary EVs as biomarkers of renal and metabolic disturbance in these individuals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.G.H. and T.M.W. have been awarded a patent (U.S. patent number 11,866,782 B2; International patent application number PCT/2017/043348) for the use of extracellular RNA to identify markers of muscular dystrophies. The remaining authors declare no competing interests.
Figures







References
-
- Turner, C. & Hilton-Jones, D. Myotonic dystrophy: diagnosis, management and new therapies. Curr. Opin. Neurol.27, 599–606 (2014). - PubMed
-
- Krentz, A. J., Williams, A. C. & Nattrass, M. Insulin resistance in multiple aspects of intermediary metabolism in myotonic dystrophy. Metabolism40, 866–872 (1991). - PubMed
-
- Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3’ end of a transcript encoding a protein kinase family member. Cell68, 799–808 (1992). - PubMed
MeSH terms
Substances
Grants and funding
- K23 NS125110/NS/NINDS NIH HHS/United States
- R61NS117210/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- W81XWH-19-1-0392/U.S. Department of Defense (United States Department of Defense)
- W81XWH-20-1-0293/U.S. Department of Defense (United States Department of Defense)
- R61 NS117210/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

