Phosphorylated tau 181 and 217 are elevated in serum and muscle of patients with amyotrophic lateral sclerosis
- PMID: 40044663
- PMCID: PMC11882981
- DOI: 10.1038/s41467-025-57144-7
Phosphorylated tau 181 and 217 are elevated in serum and muscle of patients with amyotrophic lateral sclerosis
Abstract
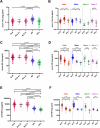
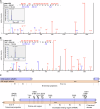
Blood phosphorylated (p)-tau 181 and p-tau 217 have been proposed as accurate biomarkers of Alzheimer's disease (AD) pathology. However, blood p-tau 181 is also elevated in amyotrophic lateral sclerosis (ALS) without a clearly identified source. We measured serum p-tau 181 and p-tau 217 in a multicentre cohort of ALS (n = 152), AD (n = 111) cases and disease controls (n = 99) recruited from four different centres. Further, we investigated the existence of both p-tau species using immunohistochemistry (IHC) and mass spectrometry (MS) in muscle biopsies of ALS cases (IHC: n = 13, MS: n = 5) and disease controls (IHC: n = 14, MS: n = 5) from one cohort. Serum p-tau 181 and p-tau 217 were higher in AD and ALS patients compared to disease controls. IHC and MS analyses revealed the presence of p-tau 181 and 217 in muscle biopsies from both ALS cases and disease controls, with ALS samples showing increased p-tau reactivity in atrophic muscle fibres. Blood p-tau species could potentially be used to diagnose both ALS and AD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.A.R. received research support from the Medical Faculty of Martin-Luther-University Halle-Wittenberg (Clinician Scientist Programm No. CS22/06), unrelated to the work presented in this paper. A.M. has received advisory board honoraria and speaking fees from Hormosan and Sanofi, all unrelated to the submitted work. J.C. received research support from the German Foundation of Neurology (Deutsche Stiftung Neurologie), the Rolf-Schwiete-Stiftung, and the programme for research and education at LMU Munich (FoeFoLe-LMU), all unrelated to the work presented in the paper. L.B. received research support from the Medical Faculty of Martin-Luther-University Halle-Wittenberg (Junior Clinician Scientist Programm No. JCS24/02), unrelated to the work presented in this paper. P.O. received research support from the Cure Alzheimer Fund, ALS Association (24-SGP-691, 23-PPG-674-2), ALS Finding a Cure, the Charcot Foundation, the DZNE Innovation-to-Application programme and consulting fees from LifeArc and Fundamental Pharma, unrelated to the work presented in this paper. V.S. received compensation for consulting services and/or speaking activities from AveXis, Cytokinetics, Italfarmaco, Liquidweb S.r.l., Novartis Pharma AG, Amylyx Pharmaceuticals, Biogen, and Zambon Biotech SA; receives or has received research supports from the Italian Ministry of Health, AriSLA, and E-Rare Joint Transnational Call, all unrelated to the work presented in this paper. V.S. is in the Editorial Board of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, European Neurology, American Journal of Neurodegenerative Diseases, Frontiers in Neurology, and Exploration of Neuroprotective Therapy. V.S., N.T. and F.V. acknowledge the support of Italian Ministry of Health (Ricerca Corrente/Ricerca Finalizzata; Hub Life Science-Diagnostica Avanzata (HLS-DA), PNC-E3-2022-23683266, the Italian Ministry of Health within the Complementary National Plan Innovative Health Ecosystem) and the Italian Ministry of Education and Research (“Dipartimenti di Eccellenza” Program 2023-2027, Department of Pathophysiology and Transplantation, Università degli Studi di Milano), unrelated to the work presented in this paper. C.S. received support from the Doris Ruess Stiftung, unrelated to the work presented in this paper. M.O. received research support from the German Federal Ministry of Education and Research (projects: FTLDc 01GI1007A), the EU Moodmarker programme (01EW2008), the ALS Association, the foundation of the state Baden-Württemberg (D.3830), Boehringer Ingelheim Ulm University BioCenter (D.5009), and the Thierry Latran Foundation and EU‐MIRIADE and the Roux-programme of the Martin Luther University Halle (Saale); M.O. received consulting fees from Biogen, Axon, Roche, and Grifols; and participates on the Biogen ATLAS trial board, all unrelated to the work presented in this paper; is a speaker of the german FTLD consortium, is involved in an unpaid role with the German Society for CSF Diagnostics and Neurochemistry, and is involved without pay with the Society for CSF Diagnostics and Neurochemistry. M.O., P.O., and S.H. are co-inventors of a patent application for using beta-synuclein measurement in blood. The other authors report no disclosures relevant to the manuscript.
Figures


References
-
- Barba, L. et al. Clinical and diagnostic implications of Alzheimer’s disease copathology in Lewy body disease. Brain. 10.1093/brain/awae203 (2024). - PubMed
-
- Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol.19, 422–433 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

