Tau filaments with the Alzheimer fold in human MAPT mutants V337M and R406W
- PMID: 40044789
- PMCID: PMC12263442
- DOI: 10.1038/s41594-025-01498-5
Tau filaments with the Alzheimer fold in human MAPT mutants V337M and R406W
Abstract
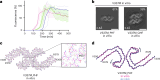
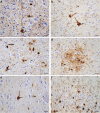
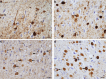
Frontotemporal dementia (FTD) and Alzheimer's disease (AD) are the most common forms of early-onset dementia. Unlike AD, FTD begins with behavioral changes before the development of cognitive impairment. Dominantly inherited mutations in MAPT, the microtubule-associated protein tau gene, give rise to cases of FTD and parkinsonism linked to chromosome 17. These individuals develop abundant filamentous tau inclusions in brain cells in the absence of β-amyloid deposits. Here, we used cryo-electron microscopy to determine the structures of tau filaments from the brains of human MAPT mutants V337M and R406W. Both amino acid substitutions gave rise to tau filaments with the Alzheimer fold, which consisted of paired helical filaments in all V337M and R406W cases and of straight filaments in two V337M cases. We also identified another assembly of the Alzheimer fold into triple tau filaments in a V337M case. Filaments assembled from recombinant tau (297-391) with substitution V337M had the Alzheimer fold and showed an increased rate of assembly.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



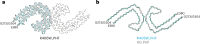




Update of
-
Tau filaments with the Alzheimer fold in cases with MAPT mutations V337M and R406W.bioRxiv [Preprint]. 2024 Apr 30:2024.04.29.591661. doi: 10.1101/2024.04.29.591661. bioRxiv. 2024. Update in: Nat Struct Mol Biol. 2025 Jul;32(7):1297-1304. doi: 10.1038/s41594-025-01498-5. PMID: 38746388 Free PMC article. Updated. Preprint.
References
-
- Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron3, 519–526 (1989). - PubMed
-
- Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci.17, 5–21 (2016). - PubMed
-
- Scheres, S. H. W., Ryskeldi-Falcon, B. & Goedert, M. Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature621, 701–710 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

