Pharmacovigilance analysis of immune checkpoint inhibitor-related reproductive adverse effects based on the FDA adverse event reporting system
- PMID: 40044844
- PMCID: PMC11883018
- DOI: 10.1038/s41598-025-91476-0
Pharmacovigilance analysis of immune checkpoint inhibitor-related reproductive adverse effects based on the FDA adverse event reporting system
Abstract
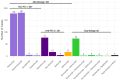
This study aims to investigate the adverse effects of immune checkpoint inhibitors (ICIs) on the female and male reproductive systems. In the FDA Adverse Event Reporting System (FAERS) database, adverse reactions under the "Reproductive system and breast disorders" category in the System Organ Classes were included, covering a period from January 1, 2015, to June 30, 2023. We identified 133,512 patients treated with ICIs. Immune checkpoint inhibitor-related reproductive adverse effects (irRAEs) were reported in 568 (0.43%) patients. Spermatogenesis abnormality (ROR025 = 7.91) had the highest signal strength associated with ICI use in males. Genital tract fistula was the only significant irRAE (ROR025 = 2.72) in females. PD-1 inhibitors pose greater risk than CTLA-4 inhibitors (OR = 1.65 [1.05-2.79], p = 0.045). Gynecologic cancers in females (OR = 3.77 [2.82-4.99], p < 0.0001) and urogenital cancers in males (OR = 1.56 [1.17-2.06], p = 0.0018) carried the highest risk compared to other cancers. Additional targeted drugs (OR = 2.32 [1.76-3.02], p < 0.0001), particularly lenvatinib (OR = 3.50 [2.48-4.94], p < 0.0001) and cabozantinib (OR = 3.71 [1.96-7.03], p < 0.0001) significantly increased the risk for females. Additional use of chemotherapy drugs was associated with a significant reduction in the risk for males (OR = 0.65 [0.42-0.96], p = 0.042) except for doxorubicin (OR = 2.58 [1.22-5.47], p = 0.013) and cyclophosphamide (OR = 2.36 [1.05-5.29], p = 0.038). This study demonstrates that ICIs could potentially lead to a wide range of adverse effects in the reproductive system in both males and females.
Keywords: Female genital system; Immune checkpoint inhibitor; Male genital system; Neoplasm.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-Related adverse events associated with immune checkpoint Blockade. N Engl. J. Med.378, 158–168 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

