Clonal dynamics and somatic evolution of haematopoiesis in mouse
- PMID: 40044850
- PMCID: PMC12074984
- DOI: 10.1038/s41586-025-08625-8
Clonal dynamics and somatic evolution of haematopoiesis in mouse
Erratum in
-
Author Correction: Clonal dynamics and somatic evolution of haematopoiesis in mouse.Nature. 2025 Oct;646(8086):E13. doi: 10.1038/s41586-025-09635-2. Nature. 2025. PMID: 40973831 Free PMC article. No abstract available.
Abstract
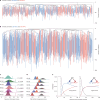
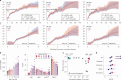
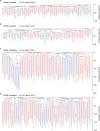
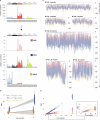
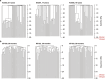
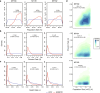
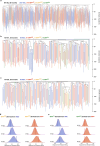
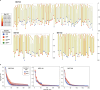
Haematopoietic stem cells maintain blood production throughout life1. Although extensively characterized using the laboratory mouse, little is known about clonal selection and population dynamics of the haematopoietic stem cell pool during murine ageing. We isolated stem cells and progenitors from young and old mice, identifying 221,890 somatic mutations genome-wide in 1,845 single-cell-derived colonies. Mouse stem cells and progenitors accrue approximately 45 somatic mutations per year, a rate only approximately threefold greater than human progenitors despite the vastly different organismal sizes and lifespans. Phylogenetic patterns show that stem and multipotent progenitor cell pools are established during embryogenesis, after which they independently self-renew in parallel over life, evenly contributing to differentiated progenitors and peripheral blood. The stem cell pool grows steadily over the mouse lifespan to about 70,000 cells, self-renewing about every 6 weeks. Aged mice did not display the profound loss of clonal diversity characteristic of human haematopoietic ageing. However, targeted sequencing showed small, expanded clones in the context of murine ageing, which were larger and more numerous following haematological perturbations, exhibiting a selection landscape similar to humans. Our data illustrate both conserved features of population dynamics of blood and distinct patterns of age-associated somatic evolution in the short-lived mouse.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






Update of
-
Clonal dynamics and somatic evolution of haematopoiesis in mouse.bioRxiv [Preprint]. 2024 Sep 21:2024.09.17.613129. doi: 10.1101/2024.09.17.613129. bioRxiv. 2024. Update in: Nature. 2025 May;641(8063):681-689. doi: 10.1038/s41586-025-08625-8. PMID: 39345649 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
- F31 HL154661/HL/NHLBI NIH HHS/United States
- R56 DK092883/DK/NIDDK NIH HHS/United States
- R01 AI141716/AI/NIAID NIH HHS/United States
- R35 HL155672/HL/NHLBI NIH HHS/United States
- F30 DK131638/DK/NIDDK NIH HHS/United States
- P01 AG036695/AG/NIA NIH HHS/United States
- F30 HD111129/HD/NICHD NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 CA183252/CA/NCI NIH HHS/United States
- U01 AG022308/AG/NIA NIH HHS/United States
- U19 AG056278/AG/NIA NIH HHS/United States
- F31 HL156500/HL/NHLBI NIH HHS/United States
- R01 CA237291/CA/NCI NIH HHS/United States
- R01 AG063543/AG/NIA NIH HHS/United States
- P01 CA265748/CA/NCI NIH HHS/United States
- R01 DK092883/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

