Aspirin prevents metastasis by limiting platelet TXA2 suppression of T cell immunity
- PMID: 40044852
- PMCID: PMC12018268
- DOI: 10.1038/s41586-025-08626-7
Aspirin prevents metastasis by limiting platelet TXA2 suppression of T cell immunity
Abstract
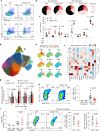
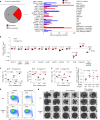
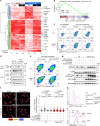
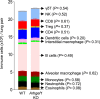
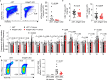
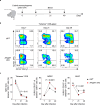
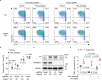
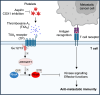
Metastasis is the spread of cancer cells from primary tumours to distant organs and is the cause of 90% of cancer deaths globally1,2. Metastasizing cancer cells are uniquely vulnerable to immune attack, as they are initially deprived of the immunosuppressive microenvironment found within established tumours3. There is interest in therapeutically exploiting this immune vulnerability to prevent recurrence in patients with early cancer at risk of metastasis. Here we show that inhibitors of cyclooxygenase 1 (COX-1), including aspirin, enhance immunity to cancer metastasis by releasing T cells from suppression by platelet-derived thromboxane A2 (TXA2). TXA2 acts on T cells to trigger an immunosuppressive pathway that is dependent on the guanine exchange factor ARHGEF1, suppressing T cell receptor-driven kinase signalling, proliferation and effector functions. T cell-specific conditional deletion of Arhgef1 in mice increases T cell activation at the metastatic site, provoking immune-mediated rejection of lung and liver metastases. Consequently, restricting the availability of TXA2 using aspirin, selective COX-1 inhibitors or platelet-specific deletion of COX-1 reduces the rate of metastasis in a manner that is dependent on T cell-intrinsic expression of ARHGEF1 and signalling by TXA2 in vivo. These findings reveal a novel immunosuppressive pathway that limits T cell immunity to cancer metastasis, providing mechanistic insights into the anti-metastatic activity of aspirin and paving the way for more effective anti-metastatic immunotherapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.R. is a scientific advisor for Enhanc3D Genomics and OligoTune Ltd and holds industrially funded collaborations with AstraZeneca PLC and F-Star Therapeutics on topics unrelated to this study. E.L. receives research grants from Bristol Meyers Squibb on a topic unrelated to this study and served as a consultant for BD Biosciences. The remaining authors declare no competing interests.
Figures











References
-
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
-
- Patrono, C., García Rodríguez, L. A., Landolfi, R. & Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med.353, 2373–2383 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

