The landscape of renal protein S-acylation in mice with lipid-induced nephrotoxicity
- PMID: 40044913
- PMCID: PMC11882957
- DOI: 10.1038/s41598-025-92530-7
The landscape of renal protein S-acylation in mice with lipid-induced nephrotoxicity
Abstract
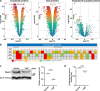
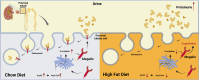
Excess fat intake is associated with kidney toxicity and dysfunction. Because fatty acids can also be reversibly attached onto cysteine residues and modulate the function of several membrane-bound proteins, we studied the effect of high-fat diet (HFD) on the S-acylated proteome of mouse kidneys to uncover novel biochemical changes that might contribute to lipid-induced nephrotoxicity. We compared the S-acylated proteome of kidneys from mice fed a chow diet (CD) or a HFD. HFD caused albuminuria. The HFD intervention induced a large-scale repression of protein S-acylation as well as of the most abundant ceramides and sphingomyelin species, which are highly suggestive of a reduction in acyl-CoA availability. The HFD-induced S-acylation repression mostly affected proteins involved in endocytosis and intracellular transport. Notably, the kidneys of mice fed a HFD displayed a marked decrease in the total amount and in the S-acylated form of megalin, the main tubular protein retrieval system. Further in vitro experiments indicated that S-acylation inhibition results in a reduction of megalin protein level. We conclude that diet-induced derangement of fatty acid metabolism modifies the renal landscape of the S-acylated proteome during the early stages of the kidney injury, which might reduce the efficiency of protein reabsorption by the proximal tubule.
Keywords: Chronic kidney disease; High-fat diet; Proteinuria; Proteomics, S-acylation; S-palmitoylation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: Experiments involving animals are described in accordance with ARRIVE guidelines.
Figures





References
-
- Levey, A. S. et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int.97, 1117–1129. 10.1016/j.kint.2020.02.010 (2020). - PubMed
-
- Jha, V. et al. Chronic kidney disease: Global dimension and perspectives. Lancet382, 260–272. 10.1016/S0140-6736(13)60687-X (2013). - PubMed
-
- Adler, A. I. et al. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int.63, 225–232. 10.1046/j.1523-1755.2003.00712.x (2003). - PubMed
-
- Wong, T. Y., Shankar, A., Klein, R. & Klein, B. E. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes53, 179–184. 10.2337/diabetes.53.1.179 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

