Balneotherapy in Fibromyalgia Syndrome: protocol of "FIBROTHERM", a prospective multi-center, two-cohort observational study
- PMID: 40044963
- PMCID: PMC12479592
- DOI: 10.1007/s00484-025-02876-w
Balneotherapy in Fibromyalgia Syndrome: protocol of "FIBROTHERM", a prospective multi-center, two-cohort observational study
Abstract
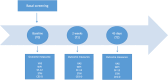
Balneotherapy (BT) is considered an effective, non-pharmacological approach, in the multimodal treatment of the Primary Fibromyalgia Syndrome (FS). However, the evidence of efficacy and tolerability of BT in FS is still limited. This is a prospective multi-center two-cohort observational study. The main aim will be the comparison of the Minimal Clinically Important Difference (according to Fibromyalgia Impact Questionnaire-FIQ) achievement in FS patients treated with BT vs standard care. Secondary objectives will be to assess: a) BT impact on pain, quality of life, anxiety and depression; b) the persistence of benefits in six weeks c) BT safety profile. All FS patients with a stable treatment in the past 3 months and a moderate to severe disease (FIQ score ≥ 39) will be enrolled after providing written informed consent. Patients will be divided into two Cohort: a) BT Cohort (i.e., BT in addition to standard care)-BTC; b) Control Cohort (i.e., only standard care)-SCC. There will be three assessments: baseline, two and six weeks (i.e., one month after BT end in BTC). At each of them the subject will fill in the following questionnaires: FIQ, VAS pain, Short Form Health Survey 16, State-Trait Anxiety Inventory and Center for Epidemiological Studies Depression Scale. We expect to observe a more relevant improvement of disease activity in BTC than in SCC. The positive effect may extend even to pain, quality of life, anxiety and depression. The short- and medium-term effects are likely to be similar, without any significant warning in terms of tolerability. Collected data, deriving from a large sample of patients, will provide a new insight of BT role in moderate to severe FS treatment. In particular, it will be possible to quantify the short and medium-term BT impact on disease activity and secondary symptoms related to FS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study will be conducted in accordance with the “Declaration of Helsinki (1964) and following amendments and approved by the Ethics Committee of Area Vasta Sud Est-Toscana (Italy) on March 2023 (decision no 23726). Consent to participate: Informed consent will be obtained from all subjects involved in the study. Consent for publication: All authors have read and agreed to the published version of the manuscript. Conflicts of interest: The Authors declare no conflicts of interest.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous