Young children (6-7 years) can meaningfully participate in cognitive interviews assessing comprehensibility in health-related quality of life domains: a qualitative study
- PMID: 40044965
- PMCID: PMC12119656
- DOI: 10.1007/s11136-025-03940-z
Young children (6-7 years) can meaningfully participate in cognitive interviews assessing comprehensibility in health-related quality of life domains: a qualitative study
Abstract
Purpose: Establishing the comprehensibility of patient reported outcome measures (PROMs) in quality of life research is essential. Cognitive interviews are recommended as a 'gold standard' for evaluating comprehensibility among adult populations but are not routinely used with young children (≤ 7 years). The current study therefore aimed to evaluate the feasibility of cognitive interviewing using traditional and adapted methods with children aged 6-7 years to evaluate PROM item comprehensibility.
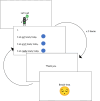
Methods: Fourteen children (6-7 years) with a range of diagnosed health conditions participated in individual cognitive interviews. Each child answered six mock PROM items (physical, psychological, and social health-related quality of life domains) and concurrent verbal probes were used to evaluate item comprehensibility. Interviews were audio recorded and transcribed verbatim. Transcripts were analysed using a novel Comprehensibility Continuum which coded the extent of alignment between children's explanations of items and intended meanings.
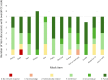
Results: Cognitive interviews were successful; extent of comprehensibility could be determined for 83/84 (99%) item discussions. Most items were comprehensible, with children describing the intended item meaning for 74/84 (88%) items evidenced by contextual examples and/or de-contextual definitions in children's responses to verbal probes. Three items ('walk', 'sad', and 'made fun of') were identified as requiring further testing and/or refinement, where a lower percentage of discussions contained evidence of intended item meaning.
Conclusion: Despite previous uncertainty, this study demonstrates how methodological challenges can be addressed to enable young children's participation in cognitive interviews evaluating item comprehensibility, ultimately contributing to the accurate measurement of young children's health outcomes in healthcare and research.
Keywords: Cognitive interview; Comprehensibility; Content validity; Patient reported outcome measures; Young children.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Philip A. Powell is an Associate Editor at Quality of Life Research. Victoria Gale and Jill Carlton declare no competing interests. The authors have no relevant financial interests to disclose. Ethical approval: Ethical approval for this research was granted by the Sheffield School of Medicine and Population Health Research Ethics Committee (Date: 02.03.2023, Reference number: 051410). Consent to participate: Written informed consent was obtained from the parents of all children included in the research. Consent for publication: The authors affirm that written informed consent was obtained from the parents of all children included in the research for the publication of anonymised participant quotes.
Figures


References
-
- Matza, L., Patrick, D., Riley, A., Alexander, J., Rajmil, L., Pleil, A., & Bullinger, M. (2013). Pediatric patient-reported outcome instruments for research to support medical product labeling: Report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value in Health,16(4), 461–479. 10.1016/j.jval.2013.04.004 - PubMed
-
- Bevans, K., Moon, J., Carle, A., Mara, C., Lai, J., DiMarco, L., Muller, N., & Woods, D. (2014). Patient reported outcomes as indicators of pediatric health care quality. Academic Pediatrics,14(5), S90–S96. 10.1016/j.acap.2014.06.002 - PubMed
-
- Ravens-Sieberer, U., Gosch, A., Abel, T., Auquier, P., Bellach, B., Bruil, J., Dür, W., Power, M., Rajmil, L., the European KIDSCREEN Group. (2001). Quality of life in children and adolescents: A European public health perspective. Sozial und Praventivmedizin,46(5), 294–302. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

