Asparagine deprivation enhances T cell antitumour response in patients via ROS-mediated metabolic and signal adaptations
- PMID: 40045118
- PMCID: PMC12116382
- DOI: 10.1038/s42255-025-01245-6
Asparagine deprivation enhances T cell antitumour response in patients via ROS-mediated metabolic and signal adaptations
Abstract
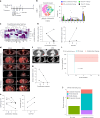
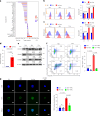
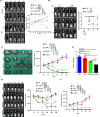
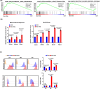
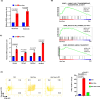
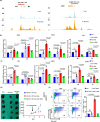
Preclinical studies have shown that asparagine deprivation enhances T cell antitumour responses. Here we apply compassionate use of L-asparaginase, usually employed to treat blood malignancies, on patients with recurrent metastatic nasopharyngeal carcinoma. The use of L-asparaginase notably enhances immune-checkpoint blockade therapy in patients by strengthening CD8+T cell fitness. Our study shows that this combination is a promising avenue for clinical application and provides further mechanistic insight into how asparagine restriction rewires T cell metabolism.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: P.-C.H. is a co-founder of Pilatus Biosciences and serves as scientific advisor board member for Pilatus Biosciences, Celyad Oncology and Elixiron Immunotherapeutics. The other authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

