Valacyclovir for the prevention of cytomegalovirus infection after kidney transplantation
- PMID: 40045190
- PMCID: PMC11881300
- DOI: 10.1186/s12879-025-10671-6
Valacyclovir for the prevention of cytomegalovirus infection after kidney transplantation
Abstract
Background: Cytomegalovirus (CMV) infection is a frequent complication after kidney transplantation (KT) and has various effects on recipient and graft survival. Although guidelines recommend anti-viral prophylaxis with ganciclovir or valganciclovir, there is a demand for alternative regimen for CMV prevention. We investigated the effects of a 3-month valacyclovir-based prophylaxis on CMV infection and clinical outcomes in KT recipients using a nationwide cohort.
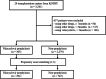
Methods: Overall, 2,584 KT recipients from 20 transplant centers registered with the Korean Organ Transplantation Registry between May 2014 and December 2019 were analyzed in this study. The recipients were divided into valacyclovir prophylaxis and non-prophylaxis groups, a 1:3 propensity score matching was performed, and 1,036 recipients (291 and 745 in the prophylaxis and non-prophylaxis groups, respectively) were analyzed. The impact of valacyclovir-based prophylaxis on CMV after KT, other clinical outcomes, and the risk factors for CMV infection development were investigated.
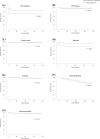
Results: The prophylaxis group showed a lower incidence of CMV infection and rejection compared to the non-prophylaxis group (3.64 vs. 10.25 events/100 person-years and 1.85 vs. 7.27 events/100 person-years, respectively). Valacyclovir prophylaxis, donor age, deceased donor, length of hospitalization after KT, anti-thymocyte globulin use, and CMV serological mismatch between the donor and recipient were independent risk factors for CMV infection after KT.
Conclusions: Valacyclovir prophylaxis after KT significantly reduced CMV infection and rejection. We suggest that valacyclovir could be considered as an alternative strategy for CMV prophylaxis after KT. However, our study has limitations, including its retrospective design, variability in valacyclovir dosing and CMV monitoring, and unassessed confounding factors. Further prospective studies with standardized protocols and larger cohorts are needed to validate our findings.
Keywords: Cytomegalovirus; Kidney transplantation; Valacyclovir.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was reviewed and approved by the Institutional Review Board of each center and the National Evidence-based Healthcare Collaborating Agency (NECA-IRB number: NECAIRB21-002). The need for informed consent was waived by the Institutional Review Board of each center (Kyung Hee University Hospital, Kyung Hee University Hospital at Gangdong, Seoul St. Mary's Hospital, Korea University Anam Hospital, Kyungpook National University Hospital, Keimyung University Dongsan Hospital, Pusan National University Hospital, Seoul National University Bundang Hospital, Seoul National University Hospital, Severance Hospital, CHA Bundang Medical Center, Samsung Medical Center, Soonchunhyang University Seoul Hospital, Inje University Busan Paik Hospital, Chonnam National University Hospital, Kangdong Sacred Heart Hospital, Incheon St. Mary's Hospital, Wonju Severance Christian Hospital, Kosin University Gospel Hospital, Eunpyeong St. Mary's Hospital) and the National Evidence-based Healthcare Collaborating Agency. Consent for publication: Not application. Competing interests: The authors declare no competing interests.
Figures
References
-
- Humar A, Snydman D, Practice ASTIDCo. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S78-86. - PubMed
-
- Brennan DC. Cytomegalovirus in renal transplantation. J Am Soc Nephrol. 2001;12(4):848–55. - PubMed
-
- Pavlopoulou ID, Syriopoulou VP, Chelioti H, Daikos GL, Stamatiades D, Kostakis A, Boletis JN. A comparative randomised study of valacyclovir vs. oral ganciclovir for cytomegalovirus prophylaxis in renal transplant recipients. Clin Microbiol Infect. 2005;11(9):736–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical