Comprehensive evaluation of tumor response better evaluates the efficacy of neoadjuvant chemotherapy and predicts the prognosis in gastric cancer - a post hoc analysis of a single-center randomized controlled trial
- PMID: 40045265
- PMCID: PMC11884205
- DOI: 10.1186/s12885-024-13372-6
Comprehensive evaluation of tumor response better evaluates the efficacy of neoadjuvant chemotherapy and predicts the prognosis in gastric cancer - a post hoc analysis of a single-center randomized controlled trial
Abstract
Background: Perioperative chemotherapy combined with D2 radical gastrectomy has been proven to be the standard treatment for local advanced gastric cancer. However, tumor regression grading (TRG) is the only neoadjuvant chemotherapy (NACT) response evaluation criterion recommended by the NCCN guideline for gastric cancer (GC). Given TRG's limitations, we aim to explore a better comprehensive response evaluation method in this study.
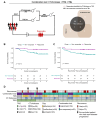
Methods: Clinical information of 96 GC patients who received NACT was collected prospectively. Clinicopathological variables predictive of the response to NACT were identified by comparing the pre- and post-NACT examination results. The correlations between the response mode and long-term survival rate were assessed.
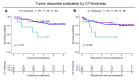
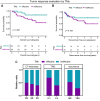
Results: Univariate Cox regression analysis showed that CT-based evaluation of the primary lesion thickness (CT-thickness) and tumor markers (TMs) were significantly associated with prognosis. The comprehensive evaluation method, including CT-thickness, TRG, and TMs, was constructed and proved to have a higher Harrell's C index. Significant differences in overall survival (OS) and recurrence-free survival (RFS) were observed between responders and non-responders distinguished by the comprehensive evaluation method.
Conclusions: The combination of CT-thickness, TRG, and TMs could be used to construct a pragmatic NACT efficacy evaluation method with both high sensitivity and specificity, which could facilitate clinical decision-making, NACT-related clinical research conduction, and efficacy predictive biomarker exploration.
Keywords: Gastric cancer; Neoadjuvant chemotherapy (NACT); Response evaluation; Tumor marker (TM); Tumor regression grading (TRG).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1964 and its later versions. The study was approved by the Peking University Cancer Hospital Ethics Committee. Informed consent was obtained from all individual participants included in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Comparison of five tumor regression grading systems for gastric adenocarcinoma after neoadjuvant chemotherapy: a retrospective study of 192 cases from National Cancer Center in China.BMC Gastroenterol. 2017 Mar 14;17(1):41. doi: 10.1186/s12876-017-0598-5. BMC Gastroenterol. 2017. PMID: 28292272 Free PMC article.
-
Efficacy and safety of XELOX combined with neoadjuvant radiotherapy versus neoadjuvant chemotherapy in locally advanced gastric cancer.BMC Cancer. 2025 Apr 18;25(1):731. doi: 10.1186/s12885-025-14103-1. BMC Cancer. 2025. PMID: 40251501 Free PMC article.
-
The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy.Gastric Cancer. 2020 May;23(3):540-549. doi: 10.1007/s10120-019-01027-6. Epub 2020 Feb 18. Gastric Cancer. 2020. PMID: 32072387 Free PMC article.
-
Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake?World J Gastroenterol. 2018 Jan 14;24(2):274-289. doi: 10.3748/wjg.v24.i2.274. World J Gastroenterol. 2018. PMID: 29375213 Free PMC article. Review.
-
Overview of adjuvant and neoadjuvant therapy for resectable gastric cancer in the East.Dig Surg. 2013;30(2):119-29. doi: 10.1159/000350877. Epub 2013 Jul 18. Dig Surg. 2013. PMID: 23867588 Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–43. - PubMed
-
- Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2011;29(13):1715–21. - PubMed
-
- Jiang L, Yang KH, Guan QL, Chen Y, Zhao P, Tian JH. Survival benefit of neoadjuvant chemotherapy for resectable cancer of the gastric and gastroesophageal junction: a meta-analysis. J Clin Gastroenterol. 2015;49(5):387–94. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

