COVID-19 lockdown-related treatment modifications did not impact the outcome of digestive cancers: the Clin-COVIDICA prospective study
- PMID: 40045328
- PMCID: PMC11881360
- DOI: 10.1186/s12885-025-13787-9
COVID-19 lockdown-related treatment modifications did not impact the outcome of digestive cancers: the Clin-COVIDICA prospective study
Abstract
Background: The Coronavirus Disease 2019 (COVID-19) pandemic modified the organization of cancer care pathways worldwide. Few prospective long-term data assessing these therapeutic modifications are available.
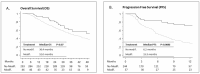
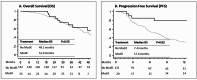
Methods: Clin-COVIDICA was a prospective cohort aiming at determining the clinical impact of COVID-19-related therapeutic modifications in patients with digestive cancer in our center. All consecutive patients undergoing an oncologic treatment for a digestive cancer from March 1 to April 30, 2020, were enrolled in the cohort and followed-up for 24 months. The primary endpoint was progression-free survival (PFS). Secondary endpoints included COVID-19 rate, adverse events (AE) and overall survival (OS). Survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test.
Results: Of the 401 patients included, 39.6% were female, mean age was 68 years old and most frequent tumor were colorectal (50.0%) and pancreatic (17.9%) cancers. All in all, 55 patients (13.7%) have undergone therapeutic modifications. The most frequent were a switch to an oral drug (capecitabine, 30.9%), treatment holidays (29.1%) and treatment cancellation (18.2%). Considering patients with palliative treatment (n = 339), there was a non-significant trend for longer OS (52.0 months versus 36.4 months, p = 0.07) and a significant longer PFS (15.4 months versus 6.2 months, p = 0.009) in patients with therapeutic modifications. There were more all grades AEs in patients without therapeutic modifications (84.4% vs. 65.5%, p = < 0.001), but more severe AEs (grade 3-5) among patients with therapeutic modifications (18.2% versus 8.7%, p = 0.048), especially for patients with a switch to an oral drug, which resulted in 8 severe adverse events and one death. Six patients (1.5%) had a COVID-19, with one COVID-19-related death and one definitive cancellation of a curative surgery due to the consequences of COVID-19.
Discussion: We observed no negative survival impact of therapeutic modifications due to the COVID-19 pandemic in digestive cancer management. This may be due to the selection of patients with less aggressive disease. More severe AEs were observed upon therapeutic modifications, especially switching to oral capecitabine.
Trial registration: Clinicaltrials.gov: NCT04389684; date of registration (15/05/2020).
Keywords: COVID-19; Capecitabine; Chemotherapy; Colorectal cancer; Treatment toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Clin-COVIDICA study was approved by a French independent Ethics Committee (Comité de Protection des Personnes Sud-Méditerranée II, IDRCB:2020-A01301-38/SI:20.04.30.59636) and registered in clinicaltrials.gov (NCT04389684). It was conducted per current French law and the ethical principles of the Helsinki Declaration of 1975 and its subsequent revisions. Before inclusion, all the patients received a written information note about the Clin-COVIDICA study. Informed consent was obtained from all subjects and/or their legal guardian; the investigators collected it. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

