Structural and functional studies of the VAPB-PTPIP51 ER-mitochondria tethering proteins in neurodegenerative diseases
- PMID: 40045432
- PMCID: PMC11881430
- DOI: 10.1186/s40478-025-01964-7
Structural and functional studies of the VAPB-PTPIP51 ER-mitochondria tethering proteins in neurodegenerative diseases
Abstract
Signaling between the endoplasmic reticulum (ER) and mitochondria regulates many of the seemingly disparate physiological functions that are damaged in neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). A number of studies have now demonstrated that ER-mitochondria signaling is perturbed in these diseases and there is evidence that this may be a driving mechanism in disease onset and progression. VAPB and PTPIP51 are ER-mitochondria tethering proteins; VAPB is an ER protein and PTPIP51 is an outer mitochondrial membrane protein and the two proteins interact to enable inter-organelle signaling. The VAPB-PTPIP51 interaction is disrupted in Alzheimer's disease, Parkinson's disease, FTD and ALS. Here we review the roles of VAPB and PTPIP51 in ER-mitochondria signaling and the mechanisms by which neurodegenerative disease insults may disrupt the VAPB-PTPIP51 interaction.
Keywords: Alzheimer’s disease; Amyotrophic lateral sclerosis; Frontotemporal dementia; Neurodegenerative diseases; Parkinson’s disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
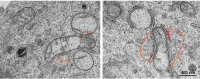

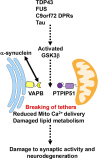
References
-
- Anagnostou G, Akbar MT, Paul P, Angelinetta C, Steiner TJ, de Belleroche J (2010) Vesicle associated membrane protein B (VAPB) is decreased in ALS spinal cord. Neurobiol Aging 31:969–985 - PubMed
-
- Anastasia I, Ilacqua N, Raimondi A, Lemieux P, Ghandehari-Alavijeh R, Faure G, Mekhedov SL, Williams KJ, Caicci F, Valle Get al et al (2021) Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Rep 34:108873. 10.1016/j.celrep.2021.108873 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

