The Potential for Twice-Annual Influenza Vaccination to Reduce Disease Burden
- PMID: 40045876
- PMCID: PMC11883281
- DOI: 10.1111/irv.70052
The Potential for Twice-Annual Influenza Vaccination to Reduce Disease Burden
Abstract
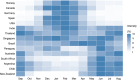
Background: Influenza vaccination is recommended annually based on the evolving nature of influenza viruses and the waning of vaccine-induced immunity. The timing of vaccination is usually before the winter influenza season in most temperate locations, where the seasonality is clear and influenza activities on average last no longer than 6 months. However, many tropical and subtropical areas have year-round influenza activity and multiple epidemics within 1 year, against which annual influenza vaccination may not offer sufficient protection at the individual level.
Aims: A twice-annual vaccination program could utilize standard inactivated influenza vaccines or enhanced influenza vaccines. Here, we discuss three reasons to consider twice-annual vaccination as a strategy to improve protection.
Discussion: The first, mentioned above, is that some locations experience prolonged or year-round influenza activity. The second reason is based on the observation that vaccine effectiveness significantly declines about 6 months after vaccination particularly for A(H3N2) strains, and therefore, vaccination twice a year might be beneficial to maintain a higher level of immunity in the second half of each year. The third reason is to allow for receipt of the most updated vaccine strains, given that these are updated twice each year by the World Health Organization. We also discuss three potential barriers or challenges. The first potential challenge is knowledge gaps, because there are very few existing studies that used twice-annual vaccination. The second potential barrier is a concern over whether more frequent vaccination would lead to reduced immunogenicity or reduced clinical protection in the longer term. The third relates to concerns about cost or feasibility.
Conclusion: We discuss these issues and recommend comparative assessment of the incremental benefits and cost of twice-annual vaccination versus annual vaccination, as well as other vaccination strategies aiming to reduce influenza disease burden particularly in tropical and subtropical locations where there can be year-round influenza activity.
Keywords: influenza vaccination; public health; vaccine effectiveness.
© 2025 The Author(s). Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
MGT has consulted for Novavax and CSL Seqirus. BJC has consulted for AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Pfizer, Roche, and Sanofi Pasteur.
Figures

References
-
- “Vaccines Against Influenza WHO Position Paper—November 2012,” Weekly Epidemiological Record 87 (2012): 461–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 IP001064/IP/NCIRD CDC HHS/United States
- HKU SRFS2021-7S03/RGC Senior Research Fellowship from the University Grants Committee of Hong Kong
- IP001064-05/US Centers for Disease Control and Prevention
- T11-712/19-N/Theme-Based Research Scheme of the Research Grants Council of the Hong Kong Special Administrative Region, China
LinkOut - more resources
Full Text Sources
Medical

