Deucravacitinib in plaque psoriasis: Four-year safety and efficacy results from the Phase 3 POETYK PSO-1, PSO-2 and long-term extension trials
- PMID: 40045918
- PMCID: PMC12188513
- DOI: 10.1111/jdv.20553
Deucravacitinib in plaque psoriasis: Four-year safety and efficacy results from the Phase 3 POETYK PSO-1, PSO-2 and long-term extension trials
Abstract
Background: Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, is approved in multiple countries for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy.
Objectives: To evaluate the safety and efficacy of deucravacitinib through 4 years in the Phase 3 POETYK PSO-1, PSO-2 and long-term extension (LTE) trials in psoriasis.
Methods: PSO-1 and PSO-2 (parent trials) randomized patients 1:2:1 to oral placebo, deucravacitinib 6 mg once daily (QD) or apremilast 30 mg twice daily. At 52 weeks, patients enrolled in the LTE trial received open-label deucravacitinib 6 mg QD. Safety was evaluated in patients who received ≥1 dose of deucravacitinib at any time. Clinical and patient-reported outcomes (PASI, PGA and DLQI) were analysed in patients who received continuous deucravacitinib from Day 1 of the parent trials and enrolled in the LTE trial.
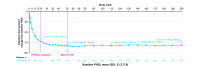
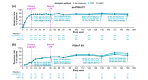
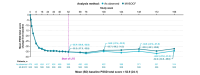
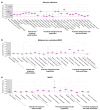
Results: In total, 1519 patients received ≥1 dose of deucravacitinib, with cumulative exposure of 4392.8 person-years (PY) through the data cut-off of 1 November 2023. Exposure-adjusted incidence rates (EAIRs)/100 PY of noted safety measures were comparable or decreased from the 1-year to 4-year cumulative period, respectively, for adverse events (AEs) (229.23, 131.68), serious AEs (including COVID-19) (5.68, 5.01), deaths (0.20, 0.25), discontinuation due to AEs (4.38, 2.20), herpes zoster (0.81, 0.55), malignancies (1.02, 0.89), major adverse cardiovascular events (0.30, 0.32) and venous thromboembolism (0.20, 0.07). In patients who received continuous deucravacitinib (n = 513), clinical and patient-reported outcome rates were well maintained from 1 year through 4 years (e.g. PASI 90, 1 year, 45.6% [95% CI, 41.3%-50.0%], 4 years, 47.5% [42.6%-52.4%]; DLQI 0/1, 1 year, 51.5% [47.1%-55.9%], 4 years, 49.4% [44.4%-54.4%]).
Conclusions: Deucravacitinib demonstrated a consistent safety profile and durable efficacy through 4 years of treatment in patients with moderate to severe plaque psoriasis.
© 2025 The Author(s). Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
Dr. Armstrong has served as a research investigator, scientific advisor and/or speaker for AbbVie, Almirall, Arcutis, Aslan, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, EPI Health, Incyte, Janssen, Leo Pharma, Lilly, Mindera Health, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma and UCB. Dr. Lebwohl has received research funds on behalf of Mount Sinai from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant, Incyte, Janssen, Lilly, Ortho Dermatologics, Regeneron and UCB, and has served as a consultant for Almirall, AltruBio, AnaptysBio, Arcutis, Avotres, Boehringer Ingelheim, Brickell Biotech, Bristol Myers Squibb, Castle Biosciences, Celltrion, CorEvitas Psoriasis Registry, Dermavant, EPI Health, Evommune, Forte Biosciences, Galderma, Genentech, Incyte, Leo Pharma, Meiji Seika Pharma, Mindera Health, Pfizer, Seanergy, Strata, Trevi and Verrica. Dr. Warren has received research grants from AbbVie, Almirall, Amgen, Celgene, Janssen, Leo Pharma, Lilly, Novartis, Pfizer and UCB, and consulting fees from AbbVie, Almirall, Amgen, Astellas, Boehringer Ingelheim, Celgene, Dice Therapeutics, GSK, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, Sanofi, UCB and Union Therapeutics. Dr. Sofen has served as a clinical investigator for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo Pharma, Lilly, Novartis and Sun Pharma. Dr. Morita has received honoraria as meeting chair/lecturer for AbbVie, AYUMI Pharmaceutical, Boehringer Ingelheim Japan, Celgene K.K., Eisai, Eli Lilly Japan K.K., Inforward, Janssen Pharmaceutical K.K., Kyowa Kirin, Maruho Co., Mitsubishi Tanabe Pharma, Nippon Kayaku, Novartis Pharma K.K., Taiho Pharmaceutical, Torii Pharmaceutical and Ushio; has received funding from AbbVie G.K., Eisai, Eli Lilly Japan K.K., Kyowa Hakko Kirin, Leo Pharma K.K., Maruho, Mitsubishi Tanabe Pharma, Novartis Pharma K.K., Taiho Pharmaceutical and Torii Pharmaceutical; and has received consulting fees from AbbVie GK, Boehringer Ingelheim Japan, Bristol Myers Squibb, Celgene K.K., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Kyowa Kirin, Maruho, Mitsubishi Tanabe Pharma, Nichi‐Iko Pharmaceutical, Nippon Kayaku, Novartis Pharma K.K., Pfizer Japan, Sun Pharma, Torii Pharmaceutical and UCB Japan. Dr. Paul has received grants and served as consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Leo Pharma, Merck, Mylan, Novartis, Pfizer, Sandoz and UCB. Dr. Papp has received honoraria and/or grants from AbbVie, Acelyrin, Akros, Alumis, Amgen, Arcutis, Bausch Health/Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Can‐Fite Biopharma, Celltrion, Concert Pharmaceuticals, Dermavant, Dermira, Dice Pharmaceuticals, Dice Therapeutics, Evelo Biosciences, Forbion, Galderma, Horizon Therapeutics, Incyte, Janssen, Kymab, Kyowa Hakko Kirin, Leo Pharma, Lilly, Meiji Seika Pharma, Mitsubishi Pharma, Nimbus Therapeutics, Novartis, Pfizer, Reistone, Sanofi‐Aventis/Genzyme, Sandoz, Sun Pharma, Takeda, Tarsus Pharmaceuticals, UCB and Zai Lab. Dr. Colombo, Ms. Scotto, Dr. Vaile, Dr. Zhou, Dr. Vritzali and Dr. Berger are employees of and stockholders in Bristol Myers Squibb. Ms. Schroeder is a consultant for Bristol Myers Squibb via Syneos Health. Dr. Banerjee was an employee of and stockholder in Bristol Myers Squibb at the time of the study. Dr. Thaçi has received research support from and a principal investigator (clinical trial funds to institution) for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Galderma, Janssen‐Cilag, Leo Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi and UCB; has served as a consultant for AbbVie, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Leo Pharma, Novartis, Pfizer and UCB; has served as a lecturer for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, Roche‐Posay, Sanofi, Target RWE and UCB; and has served on a scientific advisory board for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen‐Cilag, Leo Pharma, Lilly, Novartis, Pfizer, Sanofi and UCB. Dr. Strober has served as a consultant with honoraria for AbbVie, Acelyrin, Alamar, Almirall, Alumis, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Bristol Myers Squibb, Capital One, Celltrion, CorEvitas Psoriasis Registry, Dermavant, Imagenebio, Janssen/J&J Innovative Medicine, Kangpu Biopharmaceuticals, Leo Pharma, Lilly, Maruho, Meiji Seika Pharma, Monte Carlo, Novartis, Pfizer, Protagonist, Rapt Therapeutics, Regeneron, Sanofi, SG Cohen, Sun Pharma, Takeda, UCB, Union Therapeutics, Ventyx Biosciences and vTv Therapeutics; has served as a speaker for AbbVie, Arcutis, Dermavant, Incyte, Janssen/J&J Innovative Medicine, Lilly, Regeneron and Sanofi; has served a co‐scientific director (with consulting fee) and investigator for CorEvitas Psoriasis Registry; has served the editor‐in‐chief with an honorarium for
Figures




References
-
- Burke JR, Cheng L, Gillooly KM, Strnad J, Zupa‐Fernandez A, Catlett IM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11:eaaw1736. - PubMed
-
- Sotyktu [package insert]. Princeton, NJ: Bristol Myers Squibb; 2022.
-
- Sotyktu [European summary of product characteristics]. Dublin, Ireland: Bristol Myers Squibb EEIG; 2023.
-
- Sotyktu [package insert]. Tokyo, Japan: Bristol Myers Squibb K.K.; 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

