Non-Allergic Urticarial Skin Reactions Associated With MOv18 IgE, a First-In-Class IgE Antibody Recognising Folate Receptor Alpha
- PMID: 40045925
- PMCID: PMC12368751
- DOI: 10.1111/all.16514
Non-Allergic Urticarial Skin Reactions Associated With MOv18 IgE, a First-In-Class IgE Antibody Recognising Folate Receptor Alpha
Abstract
Background: IgE antibodies directed against cancer antigens have demonstrated potent anti-tumour effects in pre-clinical studies. MOv18 IgE, the first-in-class IgE recognising the cancer antigen folate receptor alpha (FRα), showed preliminary signs of efficacy in a Phase I trial. Treatment was well tolerated, with the most common adverse event being transient urticarial skin reactions. We investigated immunological and allergic response parameters associated with urticarial skin reactions in MOv18 IgE-treated patients.
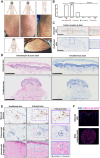
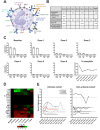
Methods: Expression of target antigen, FRα, and MOv18 IgE reactivity with FRα or any component in human skin was studied by immunohistochemistry, immunofluorescence and immuno-mass spectrometry. We conducted transcriptomic analyses in paired lesional and non-lesional skin biopsies from a patient who developed an urticarial skin reaction. Systemic immunological markers including cytokines, β-tryptase and basophil activation states were interrogated throughout the trial and contemporaneously with the skin reaction.
Results: Of the 24 IgE-treated patients, 62.5% developed transient urticarial skin reactions, with onset during the first infusion, diminishing with consecutive infusions and no β-tryptase elevation nor clinical features indicating allergic aetiology. No FRα expression or MOv18 IgE binding to human skin was identified. Lesional skin biopsies from a patient given the highest antibody dose revealed scattered eosinophils, neutrophils and mast cell degranulation, but no increased immune cell infiltration. Transcriptomic analysis indicated pro-inflammatory, but not allergic, pathway activation. No systemic allergic or hypersensitivity mediators or basophil activation were detected.
Conclusions: Urticarial skin reactions following MOv18 IgE treatment were unlikely to result from allergic mechanisms or skin antigen recognition. The clinical presentation is consistent with infusion-related reactions commonly observed with monoclonal antibody treatments.
Trial registration: EudraCT number: 2014-000070-19; ClinicalTrials.gov identifier: NCT02546921, registered 11/Sept/2015.
Keywords: AllergoOncology; IgE antibodies; folate receptor alpha; transcriptomic analysis; urticarial skin reactions.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
J.S. and S.N.K. are founders and shareholders of Epsilogen Ltd. H.J.B. is employed through a fund provided by Epsilogen Ltd. J.C. has been employed through a fund provided by Epsilogen Ltd. S.N.K., J.S., D.H.J. and H.J.B. declare patents on antibodies for cancer. R.K. is the Lead Investigator for the Phase IB/II MOv18 trial and has received travel support from Epsilogen Ltd. All other authors declare no conflicts of interest.
Figures




References
-
- Rudman S. M., Josephs D. H., Cambrook H., et al., “Harnessing Engineered Antibodies of the IgE Class to Combat Malignancy: Initial Assessment of FcvarepsilonRI‐Mediated Basophil Activation by a Tumour‐Specific IgE Antibody to Evaluate the Risk of Type I Hypersensitivity,” Clinical and Experimental Allergy 41, no. 10 (2011): 1400–1413. - PubMed
-
- Gould H. J., Mackay G. A., Karagiannis S. N., et al., “Comparison of IgE and IgG Antibody‐Dependent Cytotoxicity In Vitro and in a SCID Mouse Xenograft Model of Ovarian Carcinoma,” European Journal of Immunology 29, no. 11 (1999): 3527–3537. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 573/CU/CSP VA/United States
- 006/R/22/British Skin Foundation
- C10355/A15587/CRUK/NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre
- C7893/A29290/The Cancer Research UK City of London Centre
- C604/A25135/Cancer Research UK King's Health Partners Centre at King's College London
LinkOut - more resources
Full Text Sources
Medical

