Expanding the horizon of CAR T cell therapy: from cancer treatment to autoimmune diseases and beyond
- PMID: 40046061
- PMCID: PMC11880241
- DOI: 10.3389/fimmu.2025.1544532
Expanding the horizon of CAR T cell therapy: from cancer treatment to autoimmune diseases and beyond
Abstract
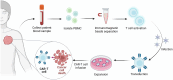
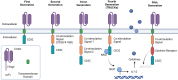
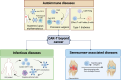
Chimeric antigen receptor (CAR)-T-cell therapy has garnered significant attention for its transformative impact on the treatment of hematologic malignancies such as leukemia and lymphoma. Despite its remarkable success, challenges such as resistance, limited efficacy in solid tumors, and adverse side effects remain prominent. This review consolidates recent advancements in CAR-T-cell therapy and explores innovative engineering techniques and strategies to overcome the immunosuppressive tumor microenvironment (TME). We also discuss emerging applications beyond cancer, including autoimmune diseases and chronic infections. Future perspectives highlight the development of more potent CAR-T cells with increased specificity and persistence and reduced toxicity, providing a roadmap for next-generation immunotherapies.
Keywords: CAR-T cells; adoptive immunotherapy; autoimmune diseases; solid tumors; tumor microenvironment.
Copyright © 2025 Yang, Ha, Wu, Ren, Yin and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fu R, Li H, Li R, McGrath K, Dotti G, Gu Z. Delivery techniques for enhancing CAR T cell therapy against solid tumors. Advanced Funct Materials. (2021) 31:2009489. doi: 10.1002/adfm.202009489 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

