Potential asthma biomarkers identified by nontargeted proteomics of extracellular vesicles in exhaled breath condensate
- PMID: 40046156
- PMCID: PMC11880581
- DOI: 10.1016/j.jacig.2025.100432
Potential asthma biomarkers identified by nontargeted proteomics of extracellular vesicles in exhaled breath condensate
Abstract
Background: Bronchial asthma (BA) and chronic obstructive pulmonary disease (COPD) are often misdiagnosed or undiagnosed, highlighting the need for more noninvasive and accessible diagnostic tools. Although exhaled breath condensate (EBC) is recognized as a biomarker resource for respiratory diseases, nontargeted proteomics of extracellular vesicles (EVs) in EBC has not been explored.
Objective: Our aim was to identify protein signatures in EBC-derived EVs (EBC-EVs) and potential biomarkers for BA and COPD.
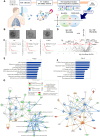
Methods: EBC-EVs were isolated from 8 patients with BA, 5 patients with COPD, and 9 healthy controls by using the phosphatidylserine affinity method. The isolated EBC-EVs were analyzed by using data-independent acquisition proteomics to identify differentially expressed proteins (DEPs) and their associations with clinical parameters.
Results: Overall, 2524 proteins were identified. In the patients with BA, 20 proteins were upregulated, and 34 were downregulated. In the patients with COPD, 46 proteins were upregulated and 67 were downregulated. Although the enriched pathways and protein networks showed similarities between BA and COPD, they also indicated distinct pathophysiologic differences. In all, 5 BA-DEPs and 2 COPD-DEPs correlated with clinical parameters. For BA, S100 calcium-binding protein P levels were inversely correlated with FEV1 value, and ribosomal protein S10 levels were inversely correlated with blood eosinophil count. Clathrin heavy chain 2 correlated with serum IgE levels. For COPD, 14-3-3 protein theta and galectin-related protein showed positive and negative correlations with FEV1 value, respectively.
Conclusions: Proteomics of EBC-EVs has enabled the identification of potential diagnostic biomarkers for BA and COPD. "Breathomics" of EBC-EVs offers a promising noninvasive approach for diagnosis and phenotyping of respiratory diseases.
Keywords: COPD; Exhaled breath condensate; bioinformatics; biomarker; bronchial asthma; endotype; exosome; extracellular vesicles; phenotype; proteomics.
© 2025 The Authors.
Conflict of interest statement
Supported by the 10.13039/501100001691Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research Program (grants JP18H05282 [to A.K.] and JP19K08650 and 22K08283 [to Y.T.]), the Japan Agency for Medical Research and Development–10.13039/501100003382Core Research for Evolutional Science and Technology (research grant 22gm1810003h0001 [to A.K.]), the Cabinet Office of Japan Government for the Public/Private R&D Investment Strategic Expansion Program, the 10.13039/100020408Kansai Economic Federation (grant to A.K.), Mitsubishi Zaidan1 (grants to A.K.), the 10.13039/100008732Uehara Memorial Foundation (grant to Y.T.), the Nippon Foundation–10.13039/501100004206Osaka University Project for Infectious Disease Prevention, the MSD Life Science Foundation (grant to Y.S.), and the 10.13039/100019085Japanese Respiratory Foundation (grant to Y.T.). Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures


References
-
- Denton E., Price D.B., Tran T.N., Canonica G.W., Menzies-Gow A., FitzGerald J.M., et al. Cluster analysis of inflammatory biomarker expression in the International Severe Asthma Registry. J Allergy Clin Immunol Pract. 2021;9:2680–2688.e7. - PubMed
-
- Aaron S.D., Vandemheen K.L., Whitmore G.A., Bergeron C., Boulet L.P., Côté A., et al. Early diagnosis and treatment of COPD and asthma—a randomized, controlled trial. N Engl J Med. 2024;390:2061–2073. - PubMed
-
- Loewenthal L., Menzies-Gow A. FeNO in asthma. Semin Respir Crit Care Med. 2022;43:635–645. - PubMed
LinkOut - more resources
Full Text Sources

