Effects of Th1/Th17 and Th2 cytokines on lipid metabolism in differentiated keratinocytes
- PMID: 40046180
- PMCID: PMC11880217
- DOI: 10.3389/fphys.2025.1387128
Effects of Th1/Th17 and Th2 cytokines on lipid metabolism in differentiated keratinocytes
Abstract
Introduction: Abnormalities of keratinocyte differentiation and impairment of permeability barrier are features of inflammatory skin diseases driven by Th1/Th17 and Th2 immune response, such as psoriasis and atopic dermatitis. We aimed at identifying the signature of the Th1/Th17 and Th2 environments on keratinocytes, focusing on the expression of genes involved in the lipid metabolism and profiles of abundance of lipid metabolites.
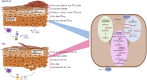
Methods: Human immortalized keratinocytes in prodifferentiative conditions induced by increasing calcium concentration, and 3D epidermal equivalents were treated with mixtures either of TNF-α and IL-17A plus Th1-related cytokines (IL-1α, IL-6) or of Th2 cytokines (IL-4, IL-13). The expression of genes involved in epidermal differentiation and lipid metabolism was evaluated by RT-PCR at 2, 4 and 7 days of treatment. The protein levels of early and late keratinocyte differentiation markers were assessed. The lipid composition was investigated by GCMS and LCMS.
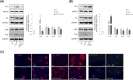
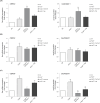
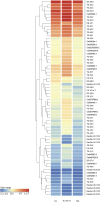
Results: Both Th1/Th17 and Th2 cytokine mixtures changed the expression of genes involved in the metabolism of fatty acids (FAs), i.e., FAS, FADS2, SCD1, and ALOX12B. Th1/ Th17 downregulated the ELOVL3 gene, which is implicated in the FAs elongation, while the mRNA levels of ABCA12 and HMGCR, genes involved in lipids transport and cholesterol synthesis, respectively, were decreased with both cytokine mixtures. DEGS1 and DEGS2, key enzymes in the ceramide synthesis, were downregulated and upregulated in the Th1/Th17 and Th2 environments, respectively. The mRNA expression of CERS3, which synthesizes ceramides containing long chain FAs, was increased by Th1/Th17 cytokines. Both Th1/ Th17 and Th2 cytokine mixtures lowered the CERS6 mRNA levels in differentiated keratinocytes. Effects specific to Th1/Th17 or Th2 cytokines were observed on freely extractable cell lipids. Th1/Th17 cytokines significantly inhibited the high calcium-induced synthesis of phospholipids (PCs, PEs, SMs), and short-chain ceramides, while the synthesis of ceramides with medium to long carbon chains was upregulated. Th2 cytokines caused a generalized decrement of free FAs, including long-chain ones. In contrast to 2D cultures, the 3D epidermal equivalents allowed the identification of altered profiles of acyland hexosyl-ceramides.
Conclusion: The different effects exerted by Th1/Th17 and Th2 cytokines support, at least in part, the features of lipid barrier alterations specific to psoriasis or atopic dermatitis.
Keywords: 3D human epidermal equivalent; Th1/Th17 cytokines; Th2 cytokines; epidermal barrier; epidermal lipids; human keratinocytes cell line.
Copyright © 2025 Cavallo, Camera, Maiellaro, Bottillo, Mosca, Kovacs, Flori and Cardinali.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

