Electrocardiographic parameter profiles for differentiating hypertrophic cardiomyopathy stages
- PMID: 40046262
- PMCID: PMC11876995
- DOI: 10.1002/joa3.70031
Electrocardiographic parameter profiles for differentiating hypertrophic cardiomyopathy stages
Abstract
Background: The efficacy of artificial intelligence (AI)-enhanced electrocardiography (ECG) for detecting hypertrophic cardiomyopathy (HCM) and its dilated phase (dHCM) has been developed, though specific ECG characteristics associated with these conditions remain insufficiently characterized.
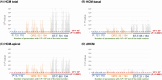
Methods: This retrospective study included 19,170 patients, with 140 HCM or dHCM cases, from the Shinken Database (2010-2017). The 140 cases (HCM-total) were categorized into basal-only HCM (HCM-basal, n = 75), apical involvement (HCM-apical, n = 46), and dHCM (n = 19). We analyzed 438 ECG parameters across the P-wave (110), QRS complex (194), and ST-T segment (134). High parameter importance (HPI) was defined as 1/p > 104 in univariate logistic regression, while multivariate logistic regression was used to determine the area under the receiver operating characteristic curves (AUROC).
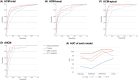
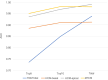
Results: In HCM-basal and HCM-apical, HPI was predominantly observed in the ST-T segment (49% and 51%, respectively), followed by the QRS complex (29% and 27%). For dHCM, HPI was lower in the ST-T segment (16%) and QRS complex (22%). The P-wave had low HPI across all subtypes. AUROCs for models with total ECG parameters were 0.925 for HCM-basal, 0.981 for HCM-apical, and 0.969 for dHCM. While AUROCs for the top 10 HPI models were lower than the total ECG parameter model for HCM total, they were comparable across HCM subtypes.
Conclusions: As HCM progresses to dHCM, a shift in HPI from the ST-T segment to the QRS complex provides clinically relevant insights. For HCM subtypes, the top 10 ECG parameters yield predictive performance similar to the full parameter set, supporting efficient approaches for AI-based diagnostic models.
Keywords: diagnostic modeling; dilated phase hypertrophic cardiomyopathy; disease progression; electrocardiogram parameters; hypertrophic cardiomyopathy.
© 2025 The Author(s). Journal of Arrhythmia published by John Wiley & Sons Australia, Ltd on behalf of Japanese Heart Rhythm Society.
Conflict of interest statement
Dr. Suzuki received lecture fees from Daiichi Sankyo and Bristol‐Myers Squibb. Dr. Yamashita received research funds and/or lecture fees from Daiichi Sankyo, Bayer Yakuhin, Bristol‐Myers Squibb, Pfizer, Nippon Boehringer Ingelheim, Eisai, Mitsubishi Tanabe Pharm, Ono Pharmaceutical, and Toa Eiyo.
Figures
References
-
- Maron BJ, Haas TS, Murphy CJ, Ahluwalia A, Rutten‐Ramos S. Incidence and causes of sudden death in U.S. college athletes. J Am Coll Cardiol. 2014;63(16):1636–1643. - PubMed
-
- Maron BJ. Risk stratification and role of implantable defibrillators for prevention of sudden death in patients with hypertrophic cardiomyopathy. Circ J. 2010;74(11):2271–2282. - PubMed
-
- Maron BJ. Clinical course and Management of Hypertrophic Cardiomyopathy. N Engl J Med. 2018;379(7):655–668. - PubMed
-
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287(10):1308–1320. - PubMed
-
- Maron BJ, Rowin EJ, Casey SA, Maron MS. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality: shaped by 50 years of clinical research and practice. JAMA Cardiol. 2016;1(1):98–105. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

