Effectiveness of a personalised self-management intervention for people living with long covid (Listen trial): pragmatic, multicentre, parallel group, randomised controlled trial
- PMID: 40046291
- PMCID: PMC11881025
- DOI: 10.1136/bmjmed-2024-001068
Effectiveness of a personalised self-management intervention for people living with long covid (Listen trial): pragmatic, multicentre, parallel group, randomised controlled trial
Abstract
Objective: To evaluate the effectiveness of Listen, a self-management support intervention, for people living with long covid who were not in hospital.
Design: Pragmatic, multicentre, parallel group, randomised controlled trial.
Setting: Twenty four sites in England and Wales.
Participants: Identified from long covid clinic waiting lists, word of mouth, and adverts/social media self-referred to the trial, 554 adults with long covid were randomised to receive either the Listen trial intervention or NHS usual care.
Interventions: The Listen intervention involved up to six one-to-one personalised sessions with trained healthcare practitioners and an accompanying handbook co-designed by people with lived experience and health professionals. Usual NHS care was variable, ranging from no access, access to mobile applications and resources, and to specialist long covid clinics.
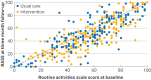
Main outcome measures: The primary outcome was the Oxford participation and activities questionnaire (Ox-PAQ) routine activities scale score at three months assessed in the intention-to-treat population. Secondary outcomes included Ox-PAQ emotional wellbeing and social engagement scale scores, the Short Form-12 (SF-12) health survey, the fatigue impact scale, and the generalised self-efficacy scale at three months. The EuroQol five-dimension five-level (EQ-5D-5L) assessed health utility. Serious adverse events were recorded.
Results: Between 27 May 2022 and 15 September 2023, 554 people with long covid (mean age 50 (standard deviation 12.3) years; 394 (72.4%) women) were randomly assigned. At three months, participants assigned to the intervention group reported small non-significant improvements in the primary outcome of capacity for daily activities as assessed by Ox-PAQ routine activities scale score (adjusted mean difference -2.68 (95% confidence interval (CI) -5.38 to 0.02), P=0.052) compared with usual NHS care. For the secondary outcomes, people receiving the intervention also reported significant improvements in mental health (Ox-PAQ emotional wellbeing -5.29 (95% CI -8.37 to -2.20), P=0.001; SF-12 2.36 (95% CI 0.77 to 3.96), P=0.004), reductions in fatigue (fatigue impact score -7.93 (95% CI -11.97 to -3.88), P<0.001), and increases in self-efficacy (generalised self-efficacy scale 2.63 (95% CI 1.50 to 3.75), P<0.001). No differences were found in social engagement (-2.07 (95% CI -5.36 to 1.22), P=0.218) or SF-12 physical health (0.32 (95% CI -0.93 to 1.57), P=0.612). No intervention related serious adverse events were reported.
Conclusions: The personalised self-management support intervention of the Listen trial resulted in non-significant short term improvements in routine activities when compared with usual care. Improvements in emotional wellbeing, fatigue, quality of life, and self-efficacy for people living with long covid were also reported. Physical health and social engagement were not affected by the trial intervention. The limited understanding of how much change is clinically meaningful in this population along with the unblinded design, the use of self-referral as a recruitment method and variable usual care may have introduced unintended bias and thus limits robust conclusions about this intervention. Further research is required to fully establish the impact of the intervention.
Trial registration number: ISRCTN36407216, ISRCTN registry, registered 27 January 2022.
Keywords: COVID-19; Clinical trial.
Copyright © Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: funding from National Institute for Health and Care Research; no support from any commercial organisation for the submitted work; no other financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work. Authors are chief investigators or co-investigators on various current research grants from the UK National Institute for Health and Care Research. The Centre for Trials Research receives infrastructure funding from Health and Care Research Wales. PRIME Centre Wales and the former Wales Covid-19 Evidence Centre (now Health & Care Research Wales Evidence Centre) receive infrastructure funding from Health and Care Research Wales. Up until March 2024 FJ's research was supported by the NIHR Applied Research Collaboration South London at King's College Hospital NHS Foundation Trust. MB was a member of the NIHR Health Technology Assessment Commissioned Funding Committee from 2020 to 2023 and is a current member of the NIHR Advanced Fellowship panel and MRC Clinical Fellowship Panel. PP is a current member of the MRC and NIHR Efficacy and Mechanism Evaluation Funding Committee. FJ is the founder and CEO of Bridges Self-Management, a nonprofit social enterprise that was involved in the co-design of the Listen intervention and training of Listen intervention practitioners. MB is recently appointed (25.04.24) as a non-executive board member of Bridges Self-Management. NS is Chief Editor, Frontiers in Health Services, Implementation Science Section.
Figures
Comment on
-
Long covid as a long term condition.BMJ Med. 2025 Jan 31;4(1):e001366. doi: 10.1136/bmjmed-2025-001366. eCollection 2025 Jan. BMJ Med. 2025. PMID: 39906532 Free PMC article. No abstract available.
References
-
- NICE guideline [NG188]. Covid-19 rapid guideline: managing the long-term effects of covid-19 [NG188] 2024. [12-Jun-2024]. www.nice.org.uk/guidance/ng188 Available. Accessed.
-
- World Health Organisation Post covid-19 condition (long covid) 2022. [12-Jun-2024]. https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-cond... Available. Accessed.
Publication types
LinkOut - more resources
Full Text Sources
