Dose Escalation Patterns and Associated Costs of Advanced Therapies for Ulcerative Colitis in France and the United Kingdom: A Retrospective Database Analysis
- PMID: 40046402
- PMCID: PMC11881627
- DOI: 10.2147/CEOR.S481730
Dose Escalation Patterns and Associated Costs of Advanced Therapies for Ulcerative Colitis in France and the United Kingdom: A Retrospective Database Analysis
Abstract
Background: Dose escalation to optimize advanced therapies is common in ulcerative colitis (UC) to avoid intra-class or inter-class drug switching and maintain clinical response and has impact on costs. Given the limited real-world data available, this study aims to understand real-world dose escalation UC advanced therapies patterns in France and United Kingdom [UK].
Methods: Retrospective study in adult patients with moderate-to-severe UC starting an advanced UC therapy (adalimumab [ADA], golimumab [GOL], infliximab [IFX], tofacitinib [TOF], ustekinumab [UST], or vedolizumab [VED]) with first prescription (and/or dispensation for France) between January 2017 and February 2022 (ie advanced UC therapy new users, by excluding patients who used any of these drugs in the previous 12 months to their index date). Proportions of patients with dose escalation/de-escalation (±20% versus Summary of Product Characteristics) after maintenance date were estimated using Kaplan-Meier (KM) survival analyses. Clinical response, healthcare resource utilization (HRU) and direct costs related to UC were also analyzed.
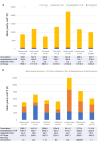
Results: Within 6 months after start of maintenance, rate of at least one dose escalation was 74.1%. Overall, 83.9-89% of patients had dose escalation within the 12-24 months, respectively, and 61.6% had clinical response [ranging from 56.3% (ADA) to 77.0% (IFX)]. Direct annual HRU costs related to UC ranged between 7426 (IFX) EUR and 22,265 (UST) in France, with mean 11,181 EUR in the dose-escalation group vs 8323 EUR in the de-escalation group (+11.5%). In the UK costs ranged between 5006 (ADA) EUR and 11,975 (UST).
Conclusion: Dose escalation of UC advanced therapies is a common strategy to avoid treatment-switching. Despite dose escalations and their cost to the system, a proportion of patients fail to achieve clinical response. This study highlights the need for more efficacious, durable treatments for moderate-to-severe UC patients, as the initiation of the advanced therapies did not reduce overall systemic/rectal corticosteroid burden.
Keywords: biologics; dose escalation; economic cost; healthcare resource use (HRU); ulcerative colitis.
© 2025 Treuer et al.
Conflict of interest statement
Dr Tamás Treuer and Dr Dhesi are employees and minor shareholders in Eli Lilly & Company, during the conduct of the study. Ms Lidia Silva reports payment made to TFS (as CRO) by contracting the data analysis and writing of respective manuscript to TFS from Eli Lilly, during the conduct of the study. The authors report no other conflicts of interest in this work. The abstract of this paper was presented at the 19th Congress of ECCO Stockholm -Sweden (on February 21–24, 2024) as a poster presentation with interim findings. The poster’s abstract was published in Volume 18, Issue Supplement_1, January 2024, Page i1667, of the Journal of Crohn’s and Colitis: https://doi.org/10.1093/ecco-jcc/jjad212.1042. A Real-World Evidence Study of Dose Escalation and Associated Costs of Advanced Therapies for Ulcerative Colitis in France and United Kingdom.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

