Drug Repurposing in Pancreatic Cancer: A Multi-Stakeholder Perspective to Improve Treatment Options for Pancreatic Cancer Patients
- PMID: 40046652
- PMCID: PMC11881603
- DOI: 10.2147/CMAR.S483151
Drug Repurposing in Pancreatic Cancer: A Multi-Stakeholder Perspective to Improve Treatment Options for Pancreatic Cancer Patients
Abstract
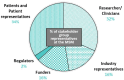
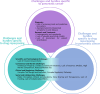
Pancreatic cancer (PC) remains one of the most challenging malignancies to treat. Current therapeutic options are unsatisfactory, and there is an urgent need for more effective and less toxic drugs to improve the dismal prognosis of PC. In recent years, drug repurposing (DR) has emerged as an attractive strategy to identify novel treatments for PC by leveraging existing drugs approved for other indications. Through the use of electronic medical records, Artificial Intelligence, study of metabolic pathways, signalling pathways, and many other approaches, it has become much easier in recent years to identify potential novel uses for old drugs. Although policy, funding and research attention in this area are steadily growing, major challenges to efficient and effective patient-centric DR in PC need to be addressed. These include but are not limited to regulatory, financial and funding barriers and the lack of coordination and collaboration among several sectors and stakeholders. To explore the opportunities and challenges associated with DR in PC, a one-day multi-stakeholder meeting was held on 14th of November 2023 in Brussels, Belgium as part of the REMEDi4ALL project. This meeting provided a platform for researchers, clinicians, industry representatives, funders, regulatory experts, and patient advocates to discuss and propose actions to optimize and accelerate DR in PC. Insights from this meeting support the potential of DR to enhance PC treatment options while highlighting the importance of systemic and supportive changes in the regulatory, policy and funding landscapes, interdisciplinary collaboration, data sharing, and patient involvement in driving therapeutic innovation. This summary highlights key outcomes and recommendations from the meeting in informing future efforts to advance DR initiatives in the context of PC.
Keywords: collaboration; drug repurposing; multi-stakeholder discussion; pancreatic cancer; patient centricity.
© 2025 Hewitt et al.
Conflict of interest statement
The views expressed in the article reflect the views of the authors and are not intended to convey the views of their employers or affiliations. The authors declare that they have no competing interest in this work.
Figures


References
-
- Pizot C, Dragomir M, Macacu A, Koechlin A, Bota M, Boyle P. Global burden of pancreas cancer: regional disparities in incidence, mortality and survival. J Health Inequalities. 2019;5:96–112. doi:10.5114/jhi.2019.87844 - DOI
-
- Wang S, Zheng R, Li J, et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol Hepatol. 2024;9:229–237. doi:10.1016/S2468-1253(23)00366-7 - DOI - PMC - PubMed
-
- Pourshams A, Sepanlou SG, Ikuta KS, et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–947. doi:10.1016/S2468-1253(19)30347-4 - DOI - PMC - PubMed
-
- American Cancer Society. Cancer Facts & Figures 2023. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- Pancreatic Cancer Across Europe. n.d Available from :https://ueg.eu/files/771/b7ee6f5f9aa5cd17ca1aea43ce84849.
LinkOut - more resources
Full Text Sources

