Disease-Associated Factors at the Endoplasmic Reticulum-Golgi Interface
- PMID: 40047103
- PMCID: PMC11883524
- DOI: 10.1111/tra.70001
Disease-Associated Factors at the Endoplasmic Reticulum-Golgi Interface
Abstract
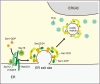
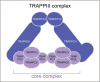
The endoplasmic reticulum (ER)-Golgi interface is essential for directing the transport of proteins synthesized in the ER to the Golgi apparatus via the ER-Golgi intermediate compartment, as well as for recycling proteins back to the ER. This transport is facilitated by various components, including COPI and COPII coat protein complexes and the transport protein particle complex. Recently, the ER-Golgi transport pathway has gained attention due to emerging evidence of nonvesicular transport mechanisms and the regulation of trafficking through liquid-liquid phase separation. Numerous diseases have been linked to mutations in proteins localized at the ER-Golgi interface, highlighting the need for comprehensive analysis of these conditions. This review examines the disease phenotypes associated with dysfunctional ER-Golgi transport factors and explores their cellular effects, providing insights into potential therapeutic strategies.
Keywords: COPI; COPII; ER exit site; ERGIC; Golgi; TRAPP complex; disease‐associated factor; endoplasmic reticulum; liquid‐phase separation; nonvesicular transport.
© 2025 The Author(s). Traffic published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

