Silk Fibroin-Based Hydrogels Supplemented with Decellularized Extracellular Matrix and Gelatin Facilitate 3D Bioprinting for Meniscus Tissue Engineering
- PMID: 40047248
- PMCID: PMC12169504
- DOI: 10.1002/mabi.202400515
Silk Fibroin-Based Hydrogels Supplemented with Decellularized Extracellular Matrix and Gelatin Facilitate 3D Bioprinting for Meniscus Tissue Engineering
Abstract
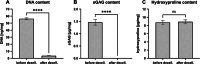
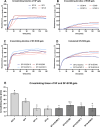
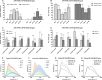
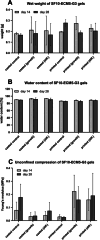
The human meniscus transmits high axial loads through the knee joint. This function is compromised upon meniscus injury or treatment by meniscectomy. 3D printing of meniscus implants has emerged as a promising alternative treatment, as it allows for precise mimicry of the meniscus architecture. In this study, silk fibroin (SF) known for its excellent mechanical properties is used to fabricate hydrogels for 3D bioprinting with infrapatellar fat pad-derived mesenchymal stem cells (IFP-MSCs). Extracellular matrix (ECM) derived from bovine menisci and gelatin are added to 10% SF to promote cell adhesion and printability. To examine the mutual influence of cells and biomaterial, experiments are conducted with and without IFP-MSCs. The cells are found to influence crosslinking, β-sheet formation, and mechanical strength. Variations between printed and casted hydrogels are identified for cell number, metabolic activity, secondary structure, and mechanical strength. Remarkably, the printed hydrogels with IFP-MSCs exhibited a compressive Young's modulus of 0.16 MPa, which closely resembled that of human osteoarthritic menisci. After initial low viability, IFP-MSCs in the casted hydrogels are able to proliferate within the biomaterial. The chondrogenic differentiation medium upregulated the expression of chondrogenic markers in the casted hydrogels, indicating promising prospects for future meniscus tissue engineering (TE).
Keywords: 3D bioprinting; meniscus tissue engineering; silk fibroin.
© 2025 The Author(s). Macromolecular Bioscience published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Masouros S. D., McDermott I. D., Amis A. A., Bull A. M. J., Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 1121. - PubMed
-
- Schwer J., Ignatius A., Seitz A. M., Acta Biomater. 2024, 175, 1. - PubMed
-
- Baratz M. E., Fu F. H., Mengato R., Am. J. Sports Med. 1986, 14, 270. - PubMed
-
- Murphy C. A., Garg A. K., Silva‐Correia J., Reis R. L., Oliveira J. M., Collins M. N., Annu. Rev. Biomed. Eng. 2019, 21, 495. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

