Sphingosine-1-phosphate signaling regulates the ability of Müller glia to become neurogenic, proliferating progenitor-like cells
- PMID: 40047533
- PMCID: PMC11884796
- DOI: 10.7554/eLife.102151
Sphingosine-1-phosphate signaling regulates the ability of Müller glia to become neurogenic, proliferating progenitor-like cells
Abstract
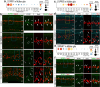
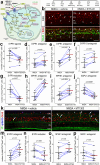
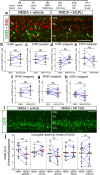
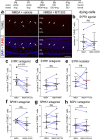
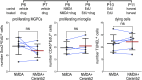
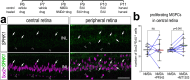
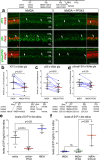
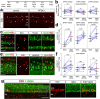
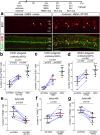
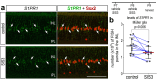
The purpose of these studies is to investigate how Sphingosine-1-phosphate (S1P) signaling regulates glial phenotype, dedifferentiation of Müller glia (MG), reprogramming into proliferating MG-derived progenitor cells (MGPCs), and neuronal differentiation of the progeny of MGPCs in the chick retina. We found that S1P-related genes are highly expressed by retinal neurons and glia, and levels of expression were dynamically regulated following retinal damage. Drug treatments that activate S1P receptor 1 (S1PR1) or increase levels of S1P suppressed the formation of MGPCs. Conversely, treatments that inhibit S1PR1 or decrease levels of S1P stimulated the formation of MGPCs. Inhibition of S1P receptors or S1P synthesis significantly enhanced the neuronal differentiation of the progeny of MGPCs. We report that S1P-related gene expression in MG is modulated by microglia and inhibition of S1P receptors or S1P synthesis partially rescues the loss of MGPC formation in damaged retinas missing microglia. Finally, we show that TGFβ/Smad3 signaling in the resting retina maintains S1PR1 expression in MG. We conclude that the S1P signaling is dynamically regulated in MG and MGPCs in the chick retina, and activation of S1P signaling depends, in part, on signals produced by reactive microglia.
Keywords: MGPCs; Müller glia; Müller glia-derived progenitor cells; S1P; Sphingosine 1 phosphate; chicken; developmental biology; regenerative medicine; retinal regeneration; stem cells.
Plain language summary
The retina is a layered structure of the central nervous system located at the back of our eyes that contains neuronal cells. Retinal neurons convert visible light into nerve signals, which travel from our eyes to our brain along the optic nerve, allowing us to see. Healthy retinas are, therefore, critical for vision. Unfortunately, many factors can damage our retinas. These range from acute eye injuries to eye diseases like diabetic retinopathy and glaucoma; if too many neurons are damaged, it can lead to blindness. In some animals, damaged retinas can repair themselves, or ‘regenerate’. This ability varies depending on the species: in fish, retinal regeneration is highly efficient, but it is reduced in birds and entirely absent in mammals – including humans. In animals that can regenerate their retinas, the resident support cells of the retina (called Müller glia) respond to retinal injury by dividing to form progenitor cells. These progenitor cells can further reprogram into new neurons to replace damaged tissue and restore sight. The efficiency of this regeneration process depends on how many cells proliferate and the ability of these progenitors to become neurons. In birds, for example, many progenitor cells are formed, but only a fraction turn into neurons. In the Müller glia of birds – specifically, in chicks – the activity of the genes for a set of biological signals (collectively termed the S1P signalling pathway, or S1P) changes after retinal injury. More generally, S1P is also known to be associated with tissue damage and inflammation. Taylor et al. therefore wanted to determine if S1P played a role in chick retinal regeneration, particularly in the development of Müller glia into ‘successful’ progenitor cells capable of replacing damaged neurons. Taylor et al. employed a combination of microscopy and genetic techniques to track the production of various cell types and measure S1P gene activity under different conditions. When chick retinas were treated with drugs known to suppress S1P activity, the production of progenitor cells increased. Importantly, these new progenitor cells were also more likely to develop into new neurons. In contrast, drugs that turned on S1P activity resulted in fewer progenitor cells being formed. These findings suggest that S1P signalling activity suppresses retinal regeneration. This study adds to our understanding of how inflammatory signals control the retina’s ability to repair itself after damage. However, more research is needed to determine the role of S1P in mammalian retinas. Ultimately, Taylor et al. hope that the knowledge gained will produce treatments to recover vision for people with retinal disease.
© 2024, Taylor et al.
Conflict of interest statement
OT, ND, HE, CG, AF No competing interests declared
Figures






Update of
-
Sphingosine-1-phosphate signaling regulates the ability of Müller glia to become neurogenic, proliferating progenitor-like cells.bioRxiv [Preprint]. 2025 Jan 24:2024.08.06.606815. doi: 10.1101/2024.08.06.606815. bioRxiv. 2025. Update in: Elife. 2025 Mar 06;13:RP102151. doi: 10.7554/eLife.102151. PMID: 39149287 Free PMC article. Updated. Preprint.
References
-
- Basavarajappa D, Gupta V, Chitranshi N, Wall RV, Rajput R, Pushpitha K, Sharma S, Mirzaei M, Klistorner A, Graham SL. Siponimod exerts neuroprotective effects on the retina and higher visual pathway through neuronal S1PR1 in experimental glaucoma. Neural Regeneration Research. 2023;18:840–848. doi: 10.4103/1673-5374.344952. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Dryad/10.5061/dryad.tdz08kq8t
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

