Modeling the Reaction Process for the Synthesis of Ethyl Chrysanthemate from Ethyl Diazoacetate in a Micro-Flow Platform
- PMID: 40047596
- PMCID: PMC11857226
- DOI: 10.3390/mi16020125
Modeling the Reaction Process for the Synthesis of Ethyl Chrysanthemate from Ethyl Diazoacetate in a Micro-Flow Platform
Abstract
Ethyl diazoacetate can react with 2,5-dimethyl-2,4-hexadiene to yield ethyl chrysanthemumate, an important raw material for synthesizing various pesticides. In conventional conditions, this cyclopropanation process suffers from low efficiency and yield due to ethyl diazoacetate. This demands more understanding of the catalytic process from the mechanism and modeling to find a solution. In this work, we set up a micro-flow platform to carefully study the kinetic characteristics of the cyclopropanation reaction of ethyl diazoacetate catalyzed by a complex of copper stearate and phenylhydrazine. Through a reasonable simplification of the reaction network, we established a reaction kinetic model with good prediction capacity within a wide range of operating conditions. It provides a basis for guiding the development of efficient conversion processes and condition optimization.
Keywords: cyclopropanation process; ethyl chrysanthemate; ethyl diazoacetate; kinetic model; reaction mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
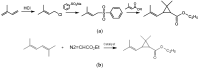








References
-
- Tessier J. Evolution of an industrial-process-deltamethrin synthesis. Chem. Ind. 1984;6:199–204.
-
- Doyle M.P., Bagheri V., Wandless T.J., Harn N.K., Brinker D.A., Eagle C.T., Loh K.L. Exceptionally high trans (anti) stereoselectivity in catalytic cyclopropanation reactions. J. Am. Chem. Soc. 1990;112:1906–1912. doi: 10.1021/ja00161a040. - DOI
-
- Pomerantz M., Rooney P. Studies on the decomposition of ethyl diazoacetate and its reaction with coal—Formation of a new tetrameric product and reagent access within the coal. Energy Fuel. 1989;3:357–361. doi: 10.1021/ef00015a017. - DOI
-
- Clark J.D., Shah A.S., Peterson J.C., Patelis L., Kersten R.J., Heemskerk A.H. Detonation properties of ethyl diazoacetate. Thermochim. Acta. 2002;386:73–79. doi: 10.1016/S0040-6031(01)00761-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

