Beyond the Lung. Impact of Elexacaftor/Tezacaftor/Ivacaftor on Sinonasal Disease in Children With Cystic Fibrosis
- PMID: 40047648
- PMCID: PMC12227108
- DOI: 10.1002/alr.23557
Beyond the Lung. Impact of Elexacaftor/Tezacaftor/Ivacaftor on Sinonasal Disease in Children With Cystic Fibrosis
Abstract
Background: Elexacaftor/tezacaftor/ivacaftor (ETI) is a Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) therapy that improves pulmonary function and chronic rhinosinusitis (CRS) in cystic fibrosis (CF) adults with at least one copy of the F508del CFTR mutation. The purpose of this study is to evaluate the impact of ETI on CRS symptoms in children and adolescents with CF.
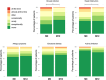
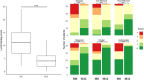
Methods: The MODUL-CF observational study is a multicenter prospective cohort study enrolling CF children with at least 1 F508del mutation in France. Subjects answered a standardized questionnaire on nasal obstruction and smell and were invited to complete the Sinus and Nasal Quality of Life Survey (SN-5) questionnaire prior to ETI therapy and at 1, 3, 6, and 12 months ETI. Part of the cohort underwent sinus computerized tomography (CT) within an ancillary study, scored by the Lund-Mackay CT score (LMKS).
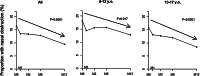
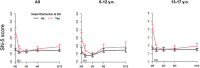
Results: Of 391 subjects, 94 (24.0%) were aged between 6 and 12 years, and 297 were adolescents. Sixty-four (16.3%) reported nasal obstruction at baseline. One hundred and sixty-three completed the SN-5 questionnaire at M0, 181 at M12, and 123 at M0 and M12. Mean SN-5 global score baseline value was similar to that of a healthy pediatric control cohort. SN-5 global score, nasal obstruction, sinus infection, and emotion domain subscores improved significantly at 1 month and this was sustained over 12 months. LMKS improved significantly in 43 patients who underwent sinus CT at M0 and M12.
Conclusion: ETI improves sinonasal symptoms and related quality of life (QOL), including emotion domain, in children and adolescents with CF.
Keywords: CFTR; CFTR modulators; chronic rhinosinusitis; cystic fibrosis; quality of life.
© 2025 The Author(s). International Forum of Allergy & Rhinology published by Wiley Periodicals LLC on behalf of American Academy of Otolaryngic Allergy and American Rhinologic Society.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

