Prostaglandin F2 receptor negative regulator as a potential target for chimeric antigen receptor-T cell therapy for glioblastoma
- PMID: 40047938
- PMCID: PMC11885767
- DOI: 10.1007/s00262-025-03979-4
Prostaglandin F2 receptor negative regulator as a potential target for chimeric antigen receptor-T cell therapy for glioblastoma
Abstract
Background: Chimeric antigen receptor (CAR)-T cell therapy targeting novel glioblastoma (GBM)-specific cell surface antigens is a promising approach. However, transcriptome analyses have revealed few GBM-specific target antigens.
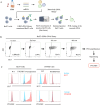
Methods: A library of monoclonal antibodies (mAbs) against tumor cell lines derived from patients with GBM was generated. mAbs reacting with tumor cells in resected tissues from patients with GBM but not with nonmalignant human brain cells were detected. The antigens that were recognized were identified through expression cloning. CAR-T cells derived from a candidate mAb were generated, and their functionality was tested in vitro and in vivo.
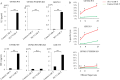
Results: Approximately 3,200 clones were established. Among them, 5E17 reacted with tumor cells in six of seven patients with GBM, but not with nonmalignant human brain cells. Prostaglandin F2 receptor negative regulator (PTGFRN) was identified as an antigen recognized by 5E17. CAR-T cells derived from 5E17 produced cytokines and exerted cytotoxicity upon co-culture with tumor cells from patients with GBM. Furthermore, intracranial injection of 5E17-CAR-T cells demonstrated antitumor effects in an orthotopic xenograft murine model with patient-derived GBM cells.
Conclusions: Cell surface PTGFRN is a candidate target for intracranial CAR-T cell therapy for GBM. On-target off-tumor toxicity in alternative normal tissues needs to be carefully tested.
Keywords: CAR-T cell therapy; Expression cloning; Glioblastoma (GBM); Monoclonal antibodies; PTGFRN.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors have no relevant financial or non-financial interests to disclose. Ethical approval: This study adhered to the tenets of the Declaration of Helsinki (2013, as amended) and was approved by the Ethics Review Committee of Osaka University Graduate School of Medicine (Suita, Osaka, Japan) (approval number: 20561). All animal experiments were approved by the Institutional Animal Care and Use Committee at Osaka University Graduate School of Medicine (approval numbers: 03–071-000 and 04–028-002) and were performed according to the animal use guidelines of the Animal Experiment Committee of Osaka University Graduate School of Medicine. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent to publish: Not applicable.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. 10.1056/NEJMoa043330 - PubMed
-
- Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z (2015) Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 314:2535–2543. 10.1001/jama.2015.16669 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

