Acute kidney injury triggers hypoxemia by lung intravascular neutrophil retention that reduces capillary blood flow
- PMID: 40048367
- PMCID: PMC12077900
- DOI: 10.1172/JCI186705
Acute kidney injury triggers hypoxemia by lung intravascular neutrophil retention that reduces capillary blood flow
Abstract
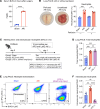
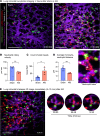
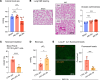
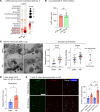
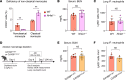
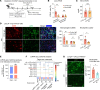
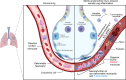
Sterile acute kidney injury (AKI) is common in the clinic and frequently associated with unexplained hypoxemia that does not improve with dialysis. AKI induces remote lung inflammation with neutrophil recruitment in mice and humans, but which cellular cues establish neutrophilic inflammation and how it contributes to hypoxemia is not known. Here we report that AKI induced rapid intravascular neutrophil retention in lung alveolar capillaries without extravasation into tissue or alveoli, causing hypoxemia by reducing lung capillary blood flow in the absence of substantial lung interstitial or alveolar edema. In contrast to direct ischemic lung injury, lung neutrophil recruitment during remote lung inflammation did not require cues from intravascular nonclassical monocytes or tissue-resident alveolar macrophages. Instead, lung neutrophil retention depended on the neutrophil chemoattractant CXCL2 released by activated classical monocytes. Comparative single-cell RNA-Seq analysis of direct and remote lung inflammation revealed that alveolar macrophages were highly activated and produced CXCL2 only in direct lung inflammation. Establishing a CXCL2 gradient into the alveolus by intratracheal CXCL2 administration during AKI-induced remote lung inflammation enabled neutrophils to extravasate. We thus discovered important differences in lung neutrophil recruitment in direct versus remote lung inflammation and identified lung capillary neutrophil retention that negatively affected oxygenation by causing a ventilation-perfusion mismatch as a driver of AKI-induced hypoxemia.
Keywords: Inflammation; Monocytes; Nephrology; Neutrophils; Pulmonology.
Conflict of interest statement
Figures

Update of
-
Acute kidney injury triggers hypoxemia by inducing intravascular neutrophil retention that reduces lung capillary blood flow.bioRxiv [Preprint]. 2025 Feb 15:2024.02.27.582396. doi: 10.1101/2024.02.27.582396. bioRxiv. 2025. Update in: J Clin Invest. 2025 Mar 6;135(10):e186705. doi: 10.1172/JCI186705. PMID: 38464306 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

