Glucagon-like peptide-1 receptor agonists, but not dipeptidyl peptidase-4 inhibitors, reduce alcohol intake
- PMID: 40048376
- PMCID: PMC12043080
- DOI: 10.1172/JCI188314
Glucagon-like peptide-1 receptor agonists, but not dipeptidyl peptidase-4 inhibitors, reduce alcohol intake
Abstract
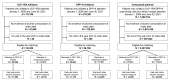
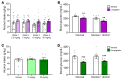
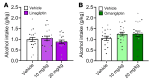
BACKGROUNDDespite growing preclinical evidence that glucagon-like peptide1 receptor agonists (GLP-1RAs) could be repurposed to treat alcohol use disorder (AUD), clinical evidence is scarce. Additionally, the potential impact of dipeptidyl peptidase-4 inhibitors (DPP-4Is) on alcohol intake is largely unknown.METHODSWe conducted a large cohort study using 2008-2023 electronic health records data from the U.S. Department of Veterans Affairs. Changes in Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) scores were compared between propensity-score-matched GLP-1RA recipients, DPP-4I recipients, and unexposed comparators. We further tested the effects of 2 DPP-4Is, linagliptin and omarigliptin, on binge-like alcohol drinking in mice and operant oral alcohol self administration in alcohol-dependent rats, models previously used to show a significant effect of the GLP-1RA semaglutide in reducing alcohol intake.RESULTSGLP-1RA recipients reported a greater reduction in AUDIT-C scores than unexposed individuals (difference-in-difference [DiD]: 0.09 [95% CI: 0.03, 0.14], P = 0.0025) and DPP-4I recipients (DiD: 0.11 [95% CI: 0.05,0.17], P = 0.0002). Reductions in drinking were more pronounced among individuals with baseline AUD (GLP-1RA versus unexposed: 0.51 [95% CI: 0.29,0.72], P < 0.0001; GLP-1RA versus DPP-4I: 0.65 [95% CI: 0.43,0.88], P < 0.0001) and baseline hazardous drinking (GLP-1RA versus unexposed: 1.38 [95% CI: 1.07,1.69], P < 0.0001; GLP-1RA versus DPP-4I: 1.00 [95% CI: 0.68,1.33], P < 0.0001). There were no differences between DPP-4I recipients and unexposed individuals. The latter results were confirmed via a reverse translational approach. Specifically, neither linagliptin nor omarigliptin reduced alcohol drinking in mice or rats. The rodent experiments also confirmed target engagemhent, as both DPP-4Is reduced blood glucose levels.CONCLUSIONConvergent findings across humans, mice, and rats indicated that GLP-1RAs, but not DPP-4Is, reduce alcohol consumption and may be efficacious in treating AUD.FUNDINGThis work was supported by the National Institutes of Health Intramural Research Program (ZIA DA000635, ZIA DA000644, ZIA DA000602), National Institute on Alcohol Abuse and Alcoholism extramural funding (R01 AA030041, P01 AA029545, U01 AA026224, U24 AA020794, U01 AA020790, U10 AA013566), the U.S. Department of Veterans Affairs (I01BX004820), and an Alkermes Pathways Research Award.
Keywords: Addiction; Endocrinology; Neuroscience; Peptides; Pharmacology.
Figures


Comment in
- GLP-1 receptor agonists for the treatment of alcohol use disorder doi: 10.1172/JCI192414
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

