Conservative endometrioma surgery: The combined technique versus CO2-laser vaporization only (BLAST: Belgium LAser STudy): Clinical protocol for a multicenter randomized controlled trial
- PMID: 40048474
- PMCID: PMC11884717
- DOI: 10.1371/journal.pone.0315709
Conservative endometrioma surgery: The combined technique versus CO2-laser vaporization only (BLAST: Belgium LAser STudy): Clinical protocol for a multicenter randomized controlled trial
Abstract
Background: The surgical management of endometrioma(s) remains challenging. Although laparoscopic surgery is a well-established treatment of endometrioma(s), caution is required to minimize ovarian damage. Several surgical techniques have been described to treat endometrioma(s): classical cystectomy, ablative techniques, or a combination of both. As cystectomy is strongly associated with a reduction in ovarian reserve, this randomized controlled trial (RCT) aims to determine to what extent the two other surgical procedures may affect ovarian reserve by comparing changes in serum anti-Müllerian hormone (AMH) levels concentrations after each type of surgery.
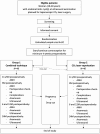
Methods: This is a multicenter, non-blinded, RCT with parallel groups (group 1 (combined technique) versus group 2 (CO2 laser vaporization only)) and allocation 1:1. Four Belgian centers will be involved. Main inclusion criteria are symptomatic patients (pain and/or infertility), 18-40 years (both inclusive) with an endometriotic cyst (mean diameter of ≥ 2.5 cm and ≤ 8 cm) and AMH level ≥ 0.7 ng/mL. Suspicion of malignancy, a contralateral endometrioma of > 2 cm, use of gonadotrophin-releasing hormone (GnRH) analogues around timing of surgery or previous oophorectomy are exclusion criteria. The primary aim is the evaluation of the difference in serum AMH levels between baseline and 3 months postoperatively (or delta AMH). The secondary outcomes include differences in AMH levels at 6 and 12 months postoperatively, cyst recurrence rate, evolution of pain pattern and fertility outcomes.
Discussion: The present study will help us to answer the question on which surgical technique for endometrioma(s) has the most favorable outcome in patients wishing to preserve their reproductive potential.
Trial registration: ClinicalTrials.gov: NCT04151433. Registered on November 5th, 2019.
Copyright: © 2025 Bafort et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: - CB: sponsorship by Gedeon Richter and Ferring Pharmaceuticals to travel and attend scientific meetings. - BG: sponsorship by Gedeon Richter, Merck SA, Ferring Pharmaceuticals and Theramex to travel and attend scientific meetings, consulting fees from Gedeon Richter. - CT: deputy director of JMIG (paid to institution, no private revenue), sponsoring by Merck SA for a clinical fellowship programme for reproductive endocrinology (paid to institution, no private revenue), consulting fees from Gedeon Richter and Merck SA (paid to institution, no private revenue), honoraria for lectures/presentations by Gedeon Richter, Merck SA and Ferring Pharmaceutical (paid to institution, no private revenue), sponsoring by Gedeon Richter, Merck SA and Ferring to travel and attend scientific meetings. - SLF, SF, MN, JLQ, LT, CW, CM: no conflict of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials