Safety run-in and part 1 of GIMEMA AML1718: venetoclax combined with FLAI as induction treatment in non-low-risk AML
- PMID: 40048742
- PMCID: PMC12148491
- DOI: 10.1182/bloodadvances.2024014901
Safety run-in and part 1 of GIMEMA AML1718: venetoclax combined with FLAI as induction treatment in non-low-risk AML
Abstract
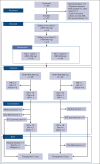
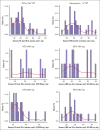
The standard induction treatment for acute myeloid leukemia (AML) has limited efficacy for patients with non-low-risk AML. We conducted a multicenter study phase 1b/2, Gruppo Italiano Malattie EMatologiche dell'Adulto AML1718, to investigate the safety and efficacy of venetoclax (VEN) combined with fludarabine, cytarabine, and idarubicin (V-FLAI) as an induction therapy for patients with non-low-risk AML aged <65 years and at intermediate or high European LeukemiaNet risk. After a safety run-in, patients were randomly allocated to VEN 400 mg or VEN 600 mg cohorts. The primary objectives were safety and composite complete remission (bone marrow blasts of <5% with any recovery). We report a predefined interim analysis after 57 patients. Median exposure to VEN during induction was 22 days. Effectiveness and safety were similar between VEN 400 mg and VEN 600 mg cohorts. The 60-day mortality rate was 5.8%. Prolonged aplasia was observed in patients receiving high doses of cytarabine during consolidation. Composite CR was achieved in 84% of patients. With a median follow-up of 20.6 months, 1-year overall survival was 71%, 1-year disease-free survival was 66.2%, and 1-year cumulative incidence of relapse was 24%. V-FLAI is an effective induction therapy for young and fit patients. Fifty-five more patients will be enrolled in part 2; they will receive VEN 400 mg + FLAI as predefined and will be evaluated centrally for measurable residual disease. This trial was registered at www.clinicaltrials.gov as #NCT03455504.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: G. Marconi acts as a consultant/speakers' bureau member of AbbVie, Astellas, AstraZeneca, Immunogen, Menarini/Stemline, Pfizer, Ryvu, Servier, Syros, and Takeda, and reports research support from AbbVie, Astellas, AstraZeneca, and Pfizer. E.A. received honoraria from AbbVie. C.P. served on an advisory board of, and/or received honoraria from, Amgen, Pfizer, Astellas, AbbVie, Blueprint, Novartis, Delbert Pharma, GlaxoSmithKline, Stemline, Incyte, Janssen, and Bristol Myers Squibb. F.G. acts as a consultant for Jazz and Astellas. M.C. received honoraria from AbbVie. G. Martinelli acts as a consultant/advisor/speakers' bureau member of Ariad/Incyte, Pfizer, Celgene/Bristol Myers Squibb, Amgen, Roche, AbbVie, GlaxoSmithKline, Astellas, Daiichi Sankyo, Takeda, and Janssen, and received research support from Pfizer, AbbVie, AstraZeneca, Daiichi Sankyo, Takeda, and Ariad/Incyte. The remaining authors declare no competing financial interests.
Figures



References
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 2022;140(12):1345–1377. - PubMed
-
- Venditti A, Piciocchi A, Candoni A, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134(12):935–945. - PubMed
-
- Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31(27):3360–3368. - PubMed
-
- Lo-Coco F, Cuneo A, Pane F, et al. Acute Leukemia Working Party of the GIMEMA group. Prognostic impact of genetic characterization in the GIMEMA LAM99P multicenter study for newly diagnosed acute myeloid leukemia. Haematologica. 2008;93(7):1017–1024. - PubMed
-
- Büchner T, Schlenk RF, Schaich M, et al. Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm–combined prospective analysis by the German AML Intergroup. J Clin Oncol. 2012;30(29):3604–3610. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

