A composite 18F-FDG PET/CT and HER2 tissue-based biomarker to predict response to neoadjuvant pertuzumab and trastuzumab in HER2-positive breast cancer (TBCRC026)
- PMID: 40049115
- PMCID: PMC11928837
- DOI: 10.1016/j.breast.2025.104432
A composite 18F-FDG PET/CT and HER2 tissue-based biomarker to predict response to neoadjuvant pertuzumab and trastuzumab in HER2-positive breast cancer (TBCRC026)
Abstract
Background: Early metabolic change on PET/CT was predictive of response to neoadjuvant trastuzumab/pertuzumab (HP) in TBCRC026. We hypothesized that a composite biomarker incorporating PET/CT and HER2 tissue-based biomarkers could improve biomarker performance.
Methods: 83 patients with estrogen receptor-negative/HER2-positive breast cancer received neoadjuvant HP alone [pathologic complete response (pCR) 22 %]. PET/CT was performed at baseline and 15 days post initiation of therapy (C1D15). Promising imaging biomarkers included ≥40 % SULmax decline between baseline and C1D15, and C1D15 SULmax ≤3. Baseline tissue-based biomarkers included HER2-enriched intrinsic subtype (72 %, 46/64; NanoString), tumor HER2 protein abundance (median log2 13.5, range log2 7.1-15.9; NanoString DSP), and HER2 3+ (83 %, 64/77; immunohistochemistry). Logistic regressions were fitted to predict pCR with HER2/PET-CT biomarkers. The C statistic assessed overall prediction power. The optimal composite score cut-off was determined by maximizing Youden's index.
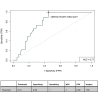
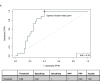
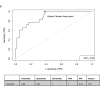
Results: Factors most predictive for pCR in single predictor models included C1D15 SULmax (OR 0.43; p = 0.007, c = 0.77), % reduction in SULmax (OR 1.03, p = 0.006, c = 0.72) and tumor HER2 protein abundance (OR 1.75; p = 0.01, c = 0.76). The composite of C1D15 SULmax and % reduction in SULmax and their interaction term, had improved probability (c = 0.89 from c = 0.78), with high sensitivity (100 %) and negative predictive value (100 %). The addition of tumor HER2 protein did not further improve prediction power (c = 0.90).
Conclusion: The HER2/PET-CT biomarker had high prediction power for pCR, however was not superior to the prediction power of PET/CT alone. Non-invasive PET/CT biomarkers may facilitate a response-guided approach to neoadjuvant therapy, allowing intensification and de-intensification of treatment, pending further evaluation.
Keywords: (18)F-FDG PET/CT; Breast cancer; HER2-Biomarkers; Neoadjuvant.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Gianni L., Pienkowski T., Im Y.H., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. - PubMed
-
- Schneeweiss A., Chia S., Hickish T., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. - PubMed
-
- Carey L.A., Berry D.A., Cirrincione C.T., et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34(6):542–549. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

