Plain Language Summary of Publication: Design of the Phase 3 AZALEA Trial of Nipocalimab in Severe Hemolytic Disease of the Fetus and Newborn
- PMID: 40049596
- PMCID: PMC12020508
- DOI: 10.1055/a-2529-4150
Plain Language Summary of Publication: Design of the Phase 3 AZALEA Trial of Nipocalimab in Severe Hemolytic Disease of the Fetus and Newborn
Abstract
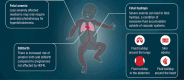
This article is a plain language summary of publication (PLSP) of the following article: Design of a phase 3, global, multicenter, randomized, placebo-controlled, double-blind study of nipocalimab in pregnancies at risk for severe hemolytic disease of the fetus and newborn by Komatsu et al published in American Journal of Perinatology on September 17, 2024 (doi:10.1055/a-2404-8089). This PLSP describes the design of the phase 3 AZALEA clinical trial in pregnant participants at risk for developing severe hemolytic disease of the fetus and newborn (HDFN). In this study, researchers will determine if an investigational treatment called nipocalimab can be used safely and effectively to treat pregnant individuals who are at risk for severe HDFN. This PLSP will help members of the public, including individuals and families affected by HDFN, understand the study. It may also be helpful for health care professionals. An infographic summary of this article is available in the Supplementary Material. · Severe HDFN causes serious health issues for babies. · IUTs carry risks of complications and fetal loss. · Nipocalimab reduces fetal anemia and IUTs in HDFN. · AZALEA is testing nipocalimab for severe HDFN.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Y.K., V.O., L.E.L., S.S.-K., M.L.T., T.V., and A.M. are employees of Janssen and hold stock/stock options from Johnson & Johnson. K.J.M. serves as the overall principal investigator for the phase 2 trial of nipocalimab (UNITY); received funding from Momenta Pharmaceuticals, Inc., paid on his behalf to the McGovern Medical School—UT Health; received funding from Janssen Pharmaceuticals, Inc., paid on his behalf to Dell Medical School at The University of Texas at Austin for a clinical trial on a monoclonal antibody for the treatment of HDFN; served on the steering committees and advisory boards for clinical studies for Momenta Pharmaceuticals, Inc., and Janssen Pharmaceuticals, Inc., but has not received funding for these activities; received royalty funding from UpToDate, Inc., for authorship of various chapters; received consulting fees from Health Management Associates, Inc., for consultation on the formation of fetal centers; received consulting fees from BillionToOne, Inc., paid on his behalf to Dell Medical School at The University of Texas at Austin; received honoraria from GLC Healthcare, Inc., for podcast content on HDFN; received payment for expert testimony/medical record review from Brinson, Askew and Berry LLP, Eckenrode and Maupin Attorneys at Law, and Parker and Poe Attorneys at Law, with fees paid to his institution; and serves as a nonpaid consultant for immunology at Janssen Pharmaceuticals, Inc.
Figures







Comment in
- Design of a Phase 3, Global, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study of Nipocalimab in Pregnancies at Risk for Severe Hemolytic Disease of the Fetus and Newborn
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

