CADOR (Capsaicin in neuropathic-like pain in digital osteoarthritis) study protocol: a multicentre randomised parallel-group trial
- PMID: 40050054
- PMCID: PMC11887280
- DOI: 10.1136/bmjopen-2024-093409
CADOR (Capsaicin in neuropathic-like pain in digital osteoarthritis) study protocol: a multicentre randomised parallel-group trial
Abstract
Introduction: It is common for finger pain in hand osteoarthritis (HOA) to display a neuropathic component. Non-steroidal anti-inflammatory drugs (NSAIDs) and conventional analgesics are not very effective in relieving this neuropathic-like pain. Capsaicin, a compound extracted from chilli peppers, is approved for the management of localised neuropathic pain. However, the effectiveness of an 8% capsaicin transdermal patch has never been tested in HOA patients with neuropathic-like pain in a randomised setting. In this study, we aimed to compare the 60-day (D60) efficacy of a transdermal application of capsaicin 8% versus a control (very low-dose capsaicin at 0.04%) on hand pain in patients with painful digital osteoarthritis with a neuropathic-like pain component.
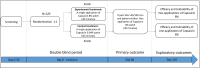
Methods and analysis: CADOR (CApsaicin in neuropathic-like pain in Digital Osteoarthritis: a Randomised trial) is a multicentre, randomised, controlled, double-blind, two parallel group, phase 3 clinical trial. Eligible patients have HOA according to the American College of Rheumatology criteria and at least Kellgren-Lawrence grade≥2, with neuropathic-like pain ('Douleur neuropathique en 4 questions' (DN4) score≥4/10 and pain intensity≥40/100 mm). At Day 0, 120 patients will be randomised (1:1) to receive a single 30 min topical application of either capsaicin 8% transdermal patch (experimental group) or capsaicin 0.04% transdermal patch (control group). The primary outcome is pain intensity in the fingers over the past 48 hours at Day 60, measured with a Visual Analogue Scale (VAS) ranging from 0 to 100 mm. Secondary outcomes are functional disability, quality of life, anxiety and depression, the patient's global impression of improvement, use of analgesics and NSAIDs and the safety of capsaicin. At visit D60, patients who still have finger pain≥40/100 mm on the VAS will receive, if they wish a single 30 min topical application of capsaicin 8% transdermal patch (not blinded). The efficacy of two transdermal applications of capsaicin (either two applications of 8% patch in the experimental arm or one application of 0.04% and one application of 8% in the control arm) will be assessed on Day 120.
Ethics and dissemination: Ethics approval was obtained from the French Health Authorities (Comité de protection des Personnes SUD EST V and Agence Nationale de Sécurité du Médicament) on 23 May 2024 in accordance with European Regulation n°536/2014 of 16 April 2014 (ref: 2024-511159-16-00) before participant enrolment.
Trial registration number: ClinicalTrials.gov: NCT06444919.
Keywords: Chronic Pain; Neurological pain; RHEUMATOLOGY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sellam J. De l’arthrose aux arthroses : une nouvelle vision phy-siopathologique. Bulletin de l’Académie Nationale de Médecine. 2018;202:139–52. doi: 10.1016/S0001-4079(19)30347-4. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous