Microglia dysfunction, neurovascular inflammation and focal neuropathologies are linked to IL-1- and IL-6-related systemic inflammation in COVID-19
- PMID: 40050441
- PMCID: PMC11893456
- DOI: 10.1038/s41593-025-01871-z
Microglia dysfunction, neurovascular inflammation and focal neuropathologies are linked to IL-1- and IL-6-related systemic inflammation in COVID-19
Abstract
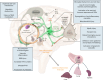
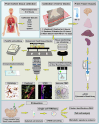
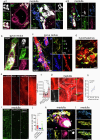
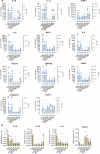
COVID-19 is associated with diverse neurological abnormalities, but the underlying mechanisms are unclear. We hypothesized that microglia, the resident immune cells of the brain, are centrally involved in this process. To study this, we developed an autopsy platform allowing the integration of molecular anatomy, protein and mRNA datasets in postmortem mirror blocks of brain and peripheral organ samples from cases of COVID-19. We observed focal loss of microglial P2Y12R, CX3CR1-CX3CL1 axis deficits and metabolic failure at sites of virus-associated vascular inflammation in severely affected medullary autonomic nuclei and other brain areas. Microglial dysfunction is linked to mitochondrial injury at sites of excessive synapse and myelin phagocytosis and loss of glutamatergic terminals, in line with proteomic changes of synapse assembly, metabolism and neuronal injury. Furthermore, regionally heterogeneous microglial changes are associated with viral load and central and systemic inflammation related to interleukin (IL)-1 or IL-6 via virus-sensing pattern recognition receptors and inflammasomes. Thus, SARS-CoV-2-induced inflammation might lead to a primarily gliovascular failure in the brain, which could be a common contributor to diverse COVID-19-related neuropathologies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures













References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

