Deep learning-driven pulmonary artery and vein segmentation reveals demography-associated vasculature anatomical differences
- PMID: 40050617
- PMCID: PMC11885638
- DOI: 10.1038/s41467-025-56505-6
Deep learning-driven pulmonary artery and vein segmentation reveals demography-associated vasculature anatomical differences
Abstract
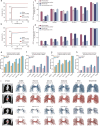
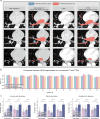
Pulmonary artery-vein segmentation is critical for disease diagnosis and surgical planning. Traditional methods rely on Computed Tomography Pulmonary Angiography (CTPA), which requires contrast agents with potential health risks. Non-contrast CT, a safer and more widely available approach, however, has long been considered impossible for this task. Here we propose High-abundant Pulmonary Artery-vein Segmentation (HiPaS), enabling accurate segmentation across both non-contrast CT and CTPA at multiple resolutions. HiPaS integrates spatial normalization with an iterative segmentation strategy, leveraging lower-level vessel segmentations as priors for higher-level segmentations. Trained on a multi-center dataset comprising 1073 CT volumes with manual annotations, HiPaS achieves superior performance (dice score: 91.8%, sensitivity: 98.0%) and demonstrates non-inferiority on non-contrast CT compared to CTPA. Furthermore, HiPaS enables large-scale analysis of 11,784 participants, revealing associations between vessel abundance and sex, age, and diseases, under lung-volume control. HiPaS represents a promising, non-invasive approach for clinical diagnostics and anatomical research.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: The patient data were collected from The First Affiliated Hospital of Harbin Medical University, the Fourth Affiliated Hospital of Harbin Medical University, Mudanjiang First People’s Hospital, China-Japan Friendship Hospital, Shanghai Renji Hospital, and Guangdong Provincial People’s Hospital, following the approval from the Institutional Review Board. The experiment using DSCTPA was approved by the Fourth Affiliated Hospital of Harbin Medical University. The study was also approved by the Institutional Biosafety and Bioethics Committee at King Abdullah University of Science and Technology. Informed consent was waived in the training cohort and the inpatient cohort due to the retrospective nature of the study. Datasets used were anonymised and any sensitive privacy information was systematically removed.
Figures


References
-
- Nabel, E. G. Cardiovascular disease. N. Engl. J. Med.349, 60–72 (2003). - PubMed
-
- Goldhaber, S. Z. & Morrison, R. B. Pulmonary embolism and deep vein thrombosis. Circulation106, 1436–1438 (2002). - PubMed
-
- McLaughlin, V. V. & McGoon, M. D. Pulmonary arterial hypertension. Circulation114, 1417–1431 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

