Evaluation of Innate Immune System, Body Habitus, and Sex on the Pharmacokinetics and Pharmacodynamics of Anetumab Ravtansine in Patients With Cancer
- PMID: 40051118
- PMCID: PMC11885412
- DOI: 10.1111/cts.70178
Evaluation of Innate Immune System, Body Habitus, and Sex on the Pharmacokinetics and Pharmacodynamics of Anetumab Ravtansine in Patients With Cancer
Abstract
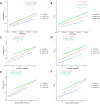
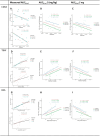
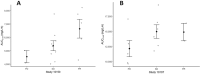
Anetumab ravtansine, like other ADC drugs, has high inter-patient variability in its pharmacokinetic (PK) and pharmacodynamic (PD) outcomes, which raises concerns about whether current dosing regimens are optimal for patients. The objective of this study was to evaluate covariates, especially body habitus and the innate immune system (IIS), which may affect anetumab ravtansine PK and PD as part of two clinical trials in patients with ovarian cancer and mesothelioma. Biomarkers of Fcγ receptors(FcγR) CD64 on IIS cells, total body weight (TBW), body surface area (BSA), and other covariates, such as sex and age, were analyzed for an association with anetumab ravtansine PK. Higher FcγR CD64, TBW, and BSA were associated with higher clearance (CL) of anetumab ravtansine (p < 0.05). However, there was no relationship between TBW or BSA and FcγR CD64. Female patients had a lower anetumab ravtansine CL (0.030 ± 0.007 L/h) compared to male patients (0.042 ± 0.006 L/h) (p < 0.05). In both studies, patients with stable disease (SD) and partial response (PR) had higher anetumab ravtansine AUC0-inf compared to patients with progressive disease (PD). Individualizing the dose of anetumab ravtansine and potentially other ADCs based only on TBW is not optimal, whereas precision dosing of an ADC based on the inclusion of novel metrics of IIS biomarkers, body habitus, and sex may be more appropriate to reduce variability in PK exposure, reduce toxicity, and improve response.
Keywords: anetumab ravtansine; antibody‐drug conjugate; body habitus; innate immune system; pharmacodynamics; pharmacokinetics; precision dosing.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
William Zamboni is the founder and CSO of Glolytics LLC, which plans to commercialize biomarkers of the innate immune system. He has also received the following funding related to this study: (1) Duke‐UNC‐Wash NCI UM1 Partnership for Early Phase Clinical Trials in Cancer (UNC Subaward 2036079/5UM1CA186704–05; REVISED PSID 5113372 IPF ID19‐2156); (2) Duke‐UNC‐Wash NCI UM1 Partnership for Early Phase Clinical Trials in Cancer—Biomarker Supplements for Assay Development/Validation and Analysis of Samples (5UM1‐CA186704‐05; PSID 5115121 IPF no. 19‐5629); and (3) UNC Lineberger Comprehensive Cancer Center Developmental Award, which is supported in part by P30 CA016086 Cancer Center Core Support Grant. Aaron Mansfield reports receiving support from Genentech and Janssen for manuscript publication; receiving research support to the institution from Novartis and Verily; receiving honoraria to the institution for participation on advisory boards for AbbVie, AstraZeneca, Bristol‐Myers Squibb, Genentech, Janssen, and Takeda Oncology; serving as a steering committee member for Janssen and Johnson & Johnson Global Services; having speaking engagements from Chugai Pharmaceutical Co. Ltd. (Roche); serving as a grant reviewer for Rising Tide; having expert think tank participation in TRIPTYCH Health Partners; serving as a moderator for Ideology Health LLC (formerly Nexus Health Media); having CME presentation for Intellisphere LLC (OncLive Summit Series) and Answers in CME; having a presentation for Immunocore; serving on the advisory board for Sanofi Genzyme; receiving honoraria to self for CME presentation for Antoni van Leeuwenhoek Kanker Instituut and MJH Life Sciences (OncLive); having presented to the University of Miami International Mesothelioma Symposium; receiving travel support from Roche; serving as a nonremunerated director of the Mesothelioma Applied Research Foundation and a member of the Friends of Patan Hospital Board of Directors; and receiving study funding and article process charges from Bristol‐Myers Squibb. He has been supported by a Mark Foundation ASPIRE Award, Thymic Carcinoma Center Research Award, Department of Defense Concept Award W81XWH‐22‐1‐0021, NCI R21 (CA251923), NCI R33 (CA272271), and NCI U24 (CA283479). The trial 10107 was supported by the NCI grants UM1 CA186709 and UM1 CA186686.
Andrew T. Lucas contributed to this manuscript while employed at the University of North Carolina, Chapel Hill. This manuscript reflects the views of the author and should not be construed to represent his new employer, PumasAI's, views or policies.
Figures

Similar articles
-
First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors.J Clin Oncol. 2020 Jun 1;38(16):1824-1835. doi: 10.1200/JCO.19.02085. Epub 2020 Mar 26. J Clin Oncol. 2020. PMID: 32213105 Free PMC article. Clinical Trial.
-
Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase Ib dose escalation and expansion study.Int J Gynecol Cancer. 2023 Apr 3;33(4):562-570. doi: 10.1136/ijgc-2022-003927. Int J Gynecol Cancer. 2023. PMID: 36564099 Free PMC article. Clinical Trial.
-
Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models.Oncotarget. 2018 Sep 25;9(75):34103-34121. doi: 10.18632/oncotarget.26135. eCollection 2018 Sep 25. Oncotarget. 2018. PMID: 30344925 Free PMC article.
-
Preclinical and clinical pharmacokinetic/pharmacodynamic considerations for antibody-drug conjugates.Expert Rev Clin Pharmacol. 2013 Sep;6(5):541-55. doi: 10.1586/17512433.2013.827405. Epub 2013 Aug 26. Expert Rev Clin Pharmacol. 2013. PMID: 23978126 Review.
-
Mesothelin Immunotherapy for Cancer: Ready for Prime Time?J Clin Oncol. 2016 Dec;34(34):4171-4179. doi: 10.1200/JCO.2016.68.3672. Epub 2016 Oct 31. J Clin Oncol. 2016. PMID: 27863199 Free PMC article. Review.
References
-
- Hassan R., Bera T., and Pastan I., “Mesothelin: A New Target for Immunotherapy,” Clinical Cancer Research 10 (2004): 3937–3942. - PubMed
-
- Dumontet C., Reichert J. M., Senter P. D., Lambert J. M., and Beck A., “Antibody‐Drug Conjugates Come of Age in Oncology,” Nature Reviews. Drug Discovery 22 (2023): 641–661. - PubMed
-
- Hassan R., G. R. Blumenschein, Jr. , Moore K. N., et al., “First‐In‐Human, Multicenter, Phase I Dose‐Escalation and Expansion Study of Anti‐Mesothelin Antibody‐Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors,” Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 38 (2020): 1824–1835. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

