Antibody-Nanoparticle Conjugates in Therapy: Combining the Best of Two Worlds
- PMID: 40051146
- PMCID: PMC12001320
- DOI: 10.1002/smll.202409635
Antibody-Nanoparticle Conjugates in Therapy: Combining the Best of Two Worlds
Abstract
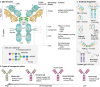
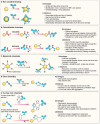
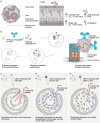
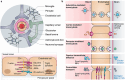
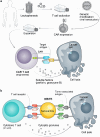
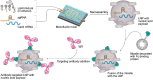
Monoclonal antibodies (mAbs) and antibody fragments have revolutionized medicine as highly specific binding agents and inhibitors. At the same time, several types of nanomaterials, including liposomes, lipid nanoparticles (NPs), polymersomes, metal and metal oxide NPs, and protein nanostructures, are increasingly utilized and explored for therapeutic potential due to their versatility, chemical and physical properties, and tunability. However, nanomaterials alone often lack specificity, leading to relatively low efficacy and/or high toxicity. To address this problem, a rapidly emerging area is antibody-nanomaterial conjugates (ANCs), which combine the precise targeting specificity of antibodies with the effector functionality of the nanomaterial. In this review, we give a brief introduction to mAbs and major conjugation techniques, describe major classes of nanomaterials being studied for therapeutic potential, and review the literature on ANCs of each class. Special focus is given to emerging applications including ANCs addressing the blood-brain barrier, ANCs delivering nucleic acids, and light-activated ANCs. While many disease targets are related to cancer, ANCs are also under development to address autoimmune, neurological, and infectious diseases. While important challenges remain, ANCs are poised to become a next-generation therapeutic technology.
Keywords: antibody; bioconjugation; liposome; mAb; nanoparticle.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
IAC is a co‐founder of Paralos Bioscience, Inc. advancing phage‐based delivery solutions.
Figures

References
-
- Strebhardt K., Ullrich A., Nat. Rev. Cancer 2008, 8, 473. - PubMed
-
- Sharmiladevi P., Girigoswami K., Haribabu V., Girigoswami A., Mater. Adv. 2021, 2, 2876.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

