A Dual-Layer Hydrogel Barrier Integrating Bio-Adhesive and Anti-Adhesive Properties Prevents Postoperative Abdominal Adhesions
- PMID: 40051152
- PMCID: PMC12023836
- DOI: 10.1002/adhm.202405238
A Dual-Layer Hydrogel Barrier Integrating Bio-Adhesive and Anti-Adhesive Properties Prevents Postoperative Abdominal Adhesions
Abstract
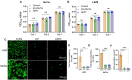
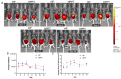
Postoperative abdominal adhesions are a common and painful complication after surgery, leading to high healthcare costs and diminished quality of life. This report presents a novel bilayer hydrogel barrier featuring an inner adhesive layer and an outer antiadhesive layer. The inner adhesive layer hydrogel (PT) is prepared by mixing polyethyleneimine (PEI) and thioctic acid (TA). The outer layer (HP) hydrogel is fabricated by the conjugation reaction of thermoresponsive zwitterionic hyaluronic acid, phenylboronic acid, and epigallocatechin gallate complex and polyvinyl alcohol based on dynamic boronic ester bond. The PEI/TA layer enhances attachment to moist tissue surfaces in vivo, and the anti-adhesive layer HP hydrogel promotes biocompatibility and anti-inflammation while minimizing protein adsorption and improving mechanical stability. The bilayer hydrogel (HPPT) exhibited rapid gelation, robust adhesion in dynamic and moist environments, superior viscoelastic properties and cellular biocompatibility. A mouse-cecum abdominal wall adhesion model is utilized to evaluate efficacy, and the HPPT hydrogel shows local retention, anti-inflammatory effect, and inhibits fibrin deposition while minimizing adhesion formation. These findings highlight the innovative structural and functional properties of the HPPT hydrogel, positioning it as a promising therapeutic barrier in peritoneal surgery aimed at reducing postoperative adhesions and enhancing surgical outcomes.
Keywords: anti‐adhesion barrier; anti‐fouling; bilayer hydrogel; zwitterionic hydrogel.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Prystowsky J. B., Stryker S. J., Ujiki G. T., Poticha S. M., Arch. Surg. 1988, 123, 855. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

