Gene therapy rescues brain edema and motor function in a mouse model of megalencephalic leukoencephalopathy with subcortical cysts
- PMID: 40051162
- PMCID: PMC11997501
- DOI: 10.1016/j.ymthe.2025.02.046
Gene therapy rescues brain edema and motor function in a mouse model of megalencephalic leukoencephalopathy with subcortical cysts
Abstract
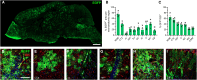
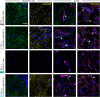
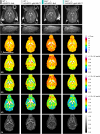
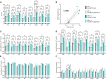
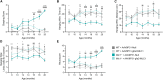
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is an ultrarare, infantile-onset leukodystrophy characterized by white matter edema for which there is no treatment. More than 75% of diagnosed cases result from biallelic loss-of-function mutations in the astrocyte-specific gene MLC1, leading to early-onset macrocephaly, cerebellar ataxia, epilepsy, and mild cognitive decline. To develop a gene therapy for MLC, we administered an adeno-associated viral vector capable of crossing the murine blood-brain barrier, delivering the human MLC1 cDNA under the control of a human astrocyte-specific promoter, to 10-month-old Mlc1-/- mice. We observed long-term astrocyte-driven expression of MLC1 up to 1 year after viral vector administration in all brain areas analyzed. Despite the late-stage intervention, in vivo magnetic resonance imaging revealed normalization of water accumulation. Notably, our therapy successfully reversed locomotor deficits in Mlc1-/- mice, as evidenced by improved performance in motor tests assessing cerebellar ataxia-like behaviors. Collectively, these findings not only demonstrate the sustained efficacy of our gene therapy but also highlight the reversibility of vacuolation and motor impairments in Mlc1-/- mice, suggesting that MLC patients could benefit from treatment even after symptom onset.
Keywords: AAV; astrocytes; cerebellum; gene therapy; leukodystrophy; megalencephalic leukoencephalopathy with subcortical cysts; myelin; neuroimaging; rare disease; white matter.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Leegwater P.A.J., Boor P.K.I., Yuan B.Q., Van Der Steen J., Visser A., Könst A.A.M., Oudejans C.B.M., Schutgens R.B.H., Pronk J.C., Van Der Knaap M.S. Identification of novel mutations in MLC1 responsible for megalencephalic leukoencephalopathy with subcortical cysts. Hum. Genet. 2002;110:279–283. doi: 10.1007/s00439-002-0682-x. - DOI - PubMed
-
- Ben-Zeev B., Levy-Nissenbaum E., Lahat H., Anikster Y., Shinar Y., Brand N., Gross-Tzur V., MacGregor D., Sidi R., Kleta R., et al. Megalencephalic leukoencephalopathy with subcortical cysts; a founder effect in Israeli patients and a higher than expected carrier rate among Libyan Jews. Hum. Genet. 2002;111:214–218. doi: 10.1007/s00439-002-0770-y. - DOI - PubMed
-
- Abdel-Salam G.M.H., Abdel-Hamid M.S., Ismail S.I., Hosny H., Omar T., Effat L., Aglan M.S., Temtamy S.A., Zaki M.S. Megalencephalic leukoencephalopathy with cysts in twelve Egyptian patients: novel mutations in MLC1 and HEPACAM and a founder effect. Metab. Brain Dis. 2016;31:1171–1179. doi: 10.1007/s11011-016-9861-7. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

