Use of artificial intelligence with retinal imaging in screening for diabetes-associated complications: systematic review
- PMID: 40052065
- PMCID: PMC11883405
- DOI: 10.1016/j.eclinm.2025.103089
Use of artificial intelligence with retinal imaging in screening for diabetes-associated complications: systematic review
Abstract
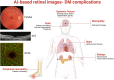
Background: Artificial Intelligence (AI) has been used to automate detection of retinal diseases from retinal images with great success, in particular for screening for diabetic retinopathy, a major complication of diabetes. Since persons with diabetes routinely receive retinal imaging to evaluate their diabetic retinopathy status, AI-based retinal imaging may have potential to be used as an opportunistic comprehensive screening for multiple systemic micro- and macro-vascular complications of diabetes.
Methods: We conducted a qualitative systematic review on published literature using AI on retina images to detect systemic diabetes complications. We searched three main databases: PubMed, Google Scholar, and Web of Science (January 1, 2000, to October 1, 2024). Research that used AI to evaluate the associations between retinal images and diabetes-associated complications, or research involving diabetes patients with retinal imaging and AI systems were included. Our primary focus was on articles related to AI, retinal images, and diabetes-associated complications. We evaluated each study for the robustness of the studies by development of the AI algorithm, size and quality of the training dataset, internal validation and external testing, and the performance. Quality assessments were employed to ensure the inclusion of high-quality studies, and data extraction was conducted systematically to gather pertinent information for analysis. This study has been registered on PROSPERO under the registration ID CRD42023493512.
Findings: From a total of 337 abstracts, 38 studies were included. These studies covered a range of topics related to prediction of diabetes from pre-diabetes or non-diabeticindividuals (n = 4), diabetes related systemic risk factors (n = 10), detection of microvascular complications (n = 8) and detection of macrovascular complications (n = 17). Most studies (n = 32) utilized color fundus photographs (CFP) as retinal image modality, while others employed optical coherence tomography (OCT) (n = 6). The performance of the AI systems varied, with an AUC ranging from 0.676 to 0.971 in prediction or identification of different complications. Study designs included cross-sectional and cohort studies with sample sizes ranging from 100 to over 100,000 participants. Risk of bias was evaluated by using the Newcastle-Ottawa Scale and AXIS, with most studies scoring as low to moderate risk.
Interpretation: Our review highlights the potential for the use of AI algorithms applied to retina images, particularly CFP, to screen, predict, or diagnose the various microvascular and macrovascular complications of diabetes. However, we identified few studies with longitudinal data and a paucity of randomized control trials, reflecting a gap between the development of AI algorithms and real-world implementation and translational studies.
Funding: Dr. Gavin Siew Wei TAN is supported by: 1. DYNAMO: Diabetes studY on Nephropathy And other Microvascular cOmplications II supported by National Medical Research Council (MOH-001327-03): data collection, analysis, trial design 2. Prognositc significance of novel multimodal imaging markers for diabetic retinopathy: towards improving the staging for diabetic retinopathy supported by NMRC Clinician Scientist Award (CSA)-Investigator (INV) (MOH-001047-00).
Keywords: Artificial intelligence; Diabetes-assciated complications; Retina image; Sereening; Systemic review.
© 2025 The Authors.
Conflict of interest statement
Prof Tien Yin Wong has received consulting fee from Aldropika Therapeutics. Bayer, Boehringer Ingelheim, Carl Zeiss, Genentech, Inc, Iveric Bio, Novartis, Opthea Limited, Quaerite Bipharm, Research Ltd, Plano, Roche, Sanofi, Shanghai Henlius. And he is a inventor, and hold patents and am a co-founder VISRE of start-up companies EyRiS and Visre, which have interests in, and develop digital solutions for eye diseases, including diabetic retinopathy. Dr Yeo Khung Keong has received research funding from Amgen, Astra Zeneca, Abbott Vascular, Bayer, Boston Scientific, Shockwave Medical, Novartis (via institution); Consulting fees from Abbott Vascular, Medtronic, Novartis, Peijia Medical; Speaker fees from Shockwave Medical, Abbott Vascular, Boston Scientific, Medtronic, Alvimedica, Biotronik, Orbus Neich, Shockwave Medical, Amgen, Novartis, Astra Zeneca, Microport, Terumo, Omnicare. Dr. Lee-Ling Lim has received Grants or contracts from: Boehringer Ingelheim, Abbott Nutrition, Zuellig Pharma Therapeutics, Novartis; and Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AstraZeneca, Boehringer Ingelheim, Abbott, Amgen, Novartis, Novo Nordisk, Roche, Sanofi, Servier, Zuellig Pharma. Dr. Aaron Y. Lee has received Grants or contracts from: Amazon, Lantham Vision Science Award, Meta, NIH/NEI K23EY029246, NIH OT2OD032644, Regeneron, Santen, Topcon, Zeiss, Research to Prevent Blindness. Consulting fees: Sanofi, Boehringer Ingelheim, Genentech, Inc., Gyroscope, Johnson & Johnson, US FDA. Dr. Daniel Ting has Patents planned, issued or pending: SELENA for DR screening altorithm Dr. Jonathan E Shaw received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Astra Zeneca, Boehringer Ingelheim, Novo Nordisk, Roche, Zuellig Pharmaceutical, Eli Lilly, Abbott. Dr. Yong Mong BEE received consulting fees from Abbott Payment and GSK, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Sebia, AstraZeneca, Boehringer Ingelheim, Participation on a Data Safety Monitoring Board or Advisory Board: Boehringer Ingelheim Honoraria paid to institution. Dr Cynthia Lim received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Sebia, AstraZeneca, Boehringer Ingelheim. Participation on a Data Safety Monitoring Board or Advisory Board: Boehringer Ingelheim Honoraria paid to institution. Dr Gavin Tan has received grants from Santen and Singapore National Research Coucil. He has received consulting and lecture fees from Novartis, Bayer, Roche, Zeiss and Leica and has received honoraria from Abbvie-Allergan, Abbott and Nikon-optos. He is also a shareholder in VISRE and Eyris.
Figures


References
-
- 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S135–S151. - PubMed
-
- Schmidt-Erfurth U., Sadeghipour A., Gerendas B.S., Waldstein S.M., Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1–29. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

